How To Determine Number Of Signals In C Nmr
13C-NMR . Spectroscopy Basics
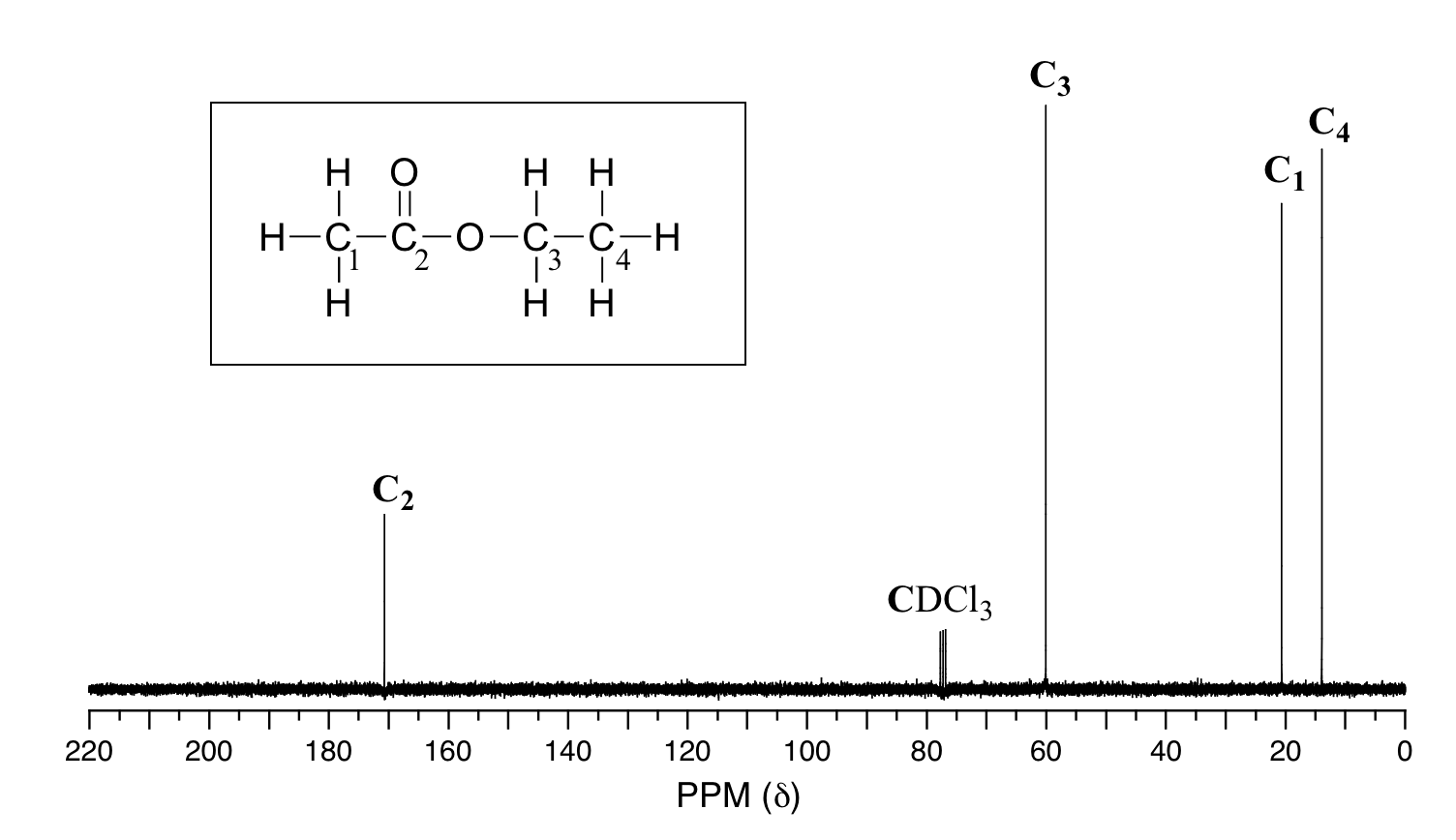
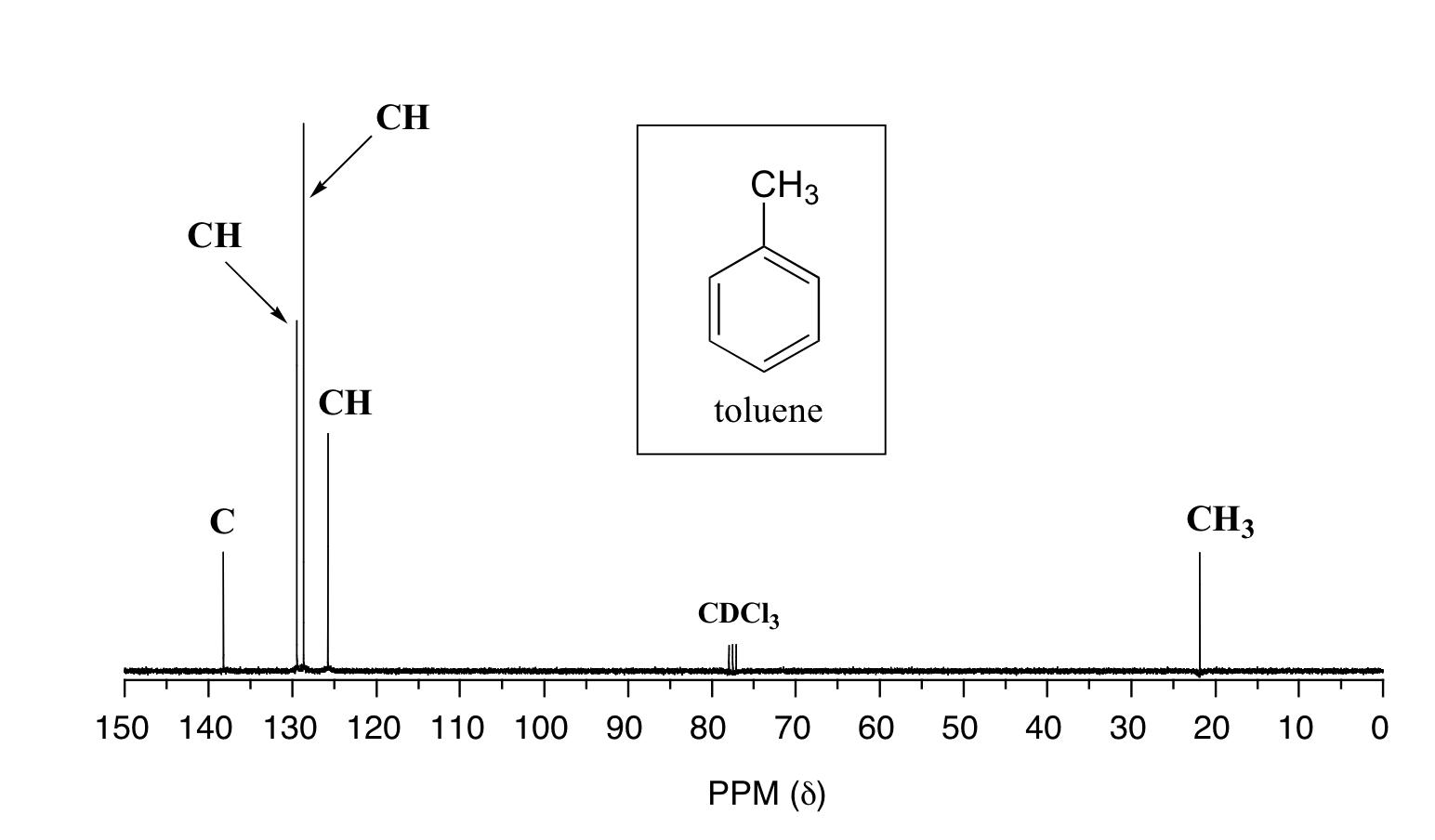
The magnetic moment of the 13C nucleus is much weaker than the magnetic moment of the proton, which means that the NMR signal from the 13C nucleus is inherently much weaker than the proton signal. This, combined with the low natural abundance of 13C, means that it is much more difficult to observe the carbon signal: more samples are needed, and often data from hundreds of scans must be averaged to yield ratios. signal-to-noise ratio to an acceptable level. Unlike the 1H-NMR signal, the region under the 13C-NMR signal cannot be used to determine the number of carbons to which it corresponds. This is because the signal for some types of carbons is inherently weaker than for others – the peaks corresponding to the carbonyl carbon atom, for example, are much smaller than the signals for the methyl or methylene peaks. (CH2). Peak integration is not usually useful in 13C-NMR spectroscopy, except when investigating molecules that have been enriched with the 13C isotope. at about 300 MHz, while the carbon resonates at about 75 MHz. This is fortunate because it allows us to look at 13C signals using a completely separate radio frequency ‘window’. Just as in the 1H-NMR, the standard used in the 13C-NMR experiments to determine the zero ppm point is tetramethylsilane (TMS), although of course in the 13C-NMR it is the signal from the four carbon equivalents. in TMS used as the standard. . The chemical change for the 13C nuclei in organic molecules is spread out over a much wider range than for protons – up to 200 ppm for 13C versus 12 ppm for protons (see Table 3 for a list). typical 13C-NMR chemical shifts). This is also fortunate, as it means that the signal from each carbon in a compound can almost always be seen as a separate peak, without the overlap that normally causes 1H-NMR spectra. The chemical shift of the 13C nuclei is influenced by essentially the same factors that affect the chemical transition of the proton: bonding with electronegative atoms and a tendency toward antimagnetic anisotropy effects. shift the signal down the field (higher resonant frequency). In addition, sp2 hybridization leads to a large downlink shift. The 13C-NMR signals for the carbonyl atom are usually the farthest down field (170-220 ppm), due to both sp2 hybridization and the oxygen double bond. For 13C nuclei, it is very difficult to find two close 13C atoms in the same molecule, and therefore we do not see spin-spin bonding between neighboring atoms in the 13C-NMR spectrum. However, there nuclear coupling between the 13C carbons and the hydrogens to which they are bonded. The carbon-proton coupling constant is very large, on the order of 100 – 250 Hz. For clarity, chemists often use a technique known as split broadband, which essentially ‘turns off’ the CH coupling, resulting in a spectrum where all the carbon signals are single signals. Below is a protonated 13C-NMR spectrum of ethyl acetate, showing four expected signals, one for each carbon atom.


Last, Wallx.net sent you details about the topic “How To Determine Number Of Signals In C Nmr❤️️”.Hope with useful information that the article “How To Determine Number Of Signals In C Nmr” It will help readers to be more interested in “How To Determine Number Of Signals In C Nmr [ ❤️️❤️️ ]”.
Posts “How To Determine Number Of Signals In C Nmr” posted by on 2021-10-27 08:35:08. Thank you for reading the article at wallx.net