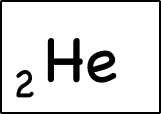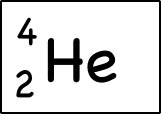Drawing Atoms
This year, I’m basing my middle school student’s introduction to basic chemistry around the periodic table of the elements. However, the first step is to teach them how to draw basic models of atoms. Preparation: Memorizing Winter Break I started by having students memorize the first 20 elements (H through Ca), in their correct order – by atomic number – during winter break.Oxygen Atom Diagrams So, to give them a bit of context, I looked at the basic parts of an atom (protons, neutrons and electrons) and made it clear that the name of the element is determined only by the number of protons . I even had them draw a few atoms with protons and neutrons in the center and electrons in the shells. Since I poured all of this on them in a single class, it’s probably a bit much, but since it’s just to give them some context, I don’t expect 7th graders who Haven’t seen this before, will remember. all; For 8th graders, this should have been just a review. Most of the students did well on the memorization section. Some found songs on the internet helped, while others just got you through. Having two weeks of winter break to do that might also help. Day 1. Lesson: Parts of an Atom When we go back to school, the first thing I do is give the children an outline of the upper part of the periodic table and ask them to fill in the element names. there.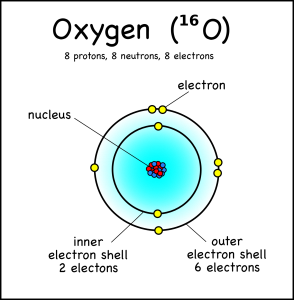
The atomic number is written as a sub-symbol to the left of the element symbol.The atomic number is the number of protons. Since they’ve memorized the elements in order, they should be able to figure this out for themselves – but they can also do a quick lookup on the periodic table or look at the element symbol where the atomic number is sometimes written in bottom left .
- Atoms have the same number of electrons as protons. Protons carry a positive charge and electrons carry a negative charge, so an atom needs to have the same amount of both for its charge to be balanced. We don’t talk about ions – where there are more or less electrons – until much later.
Atomic mass (4) is written as the symbol on the left side of the element symbol. Atomic mass is the sum of the number of protons (2) and the number of neutrons (2). The small atoms we are looking at tend to have the same number of neutrons as protons, but not necessarily. So how do you know how many neutrons there are? You have to ask, or look at the atomic mass number, which is usually written on the top left of the atom. Since atomic mass is the sum of the number of protons and neutrons, if you know the atomic mass and the number of protons you can easily find out the number of neutrons. (Note that electrons do not contribute to the mass of an atom because their mass is much smaller than that of neutrons and protons.
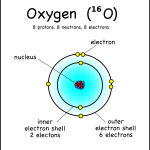
This oxygen atom has 8 electrons in two shells.Electronic case: Electrons orbit the nucleus in a series of shells. Each shell can hold a certain maximum number of electrons (2 for the first shell; 8 for the second shell; and 8 for the third). And to draw atoms, you fill the inner shells first and then move on to the outer shells.
So, if I just write the element symbol and its atomic mass on the board, the students will be able to figure out the number of particles.
Example: Carbon-12
Contents
Read more: how to remove old tape from wood For example, the most common form (isotope) of carbon-12 is written as:
- Proton = 6: Since we know the atomic number is 6 (because we have memorized it) the atom has 6 protons.
- Neutrons = 6 : Since the atomic mass is 12 (upper left of the element symbol), to find the number of neutrons we subtract the number of protons (12 – 6 = 6).
- Electrons = 6: This atom is electrically balanced so it needs their six negatively charged electrons to offset the six positive charges of the six protons. (Note: we’re not talking about charged, unbalanced atoms yet, but the charge will show up as the symbol above to the right of the symbol.)
- Electronic Case (2-4): We have six electrons, so the first two electrons go in to fill the first electron shell, and the rest can go into the second shell, which can hold up to 8 electrons. This gives an electron configuration of 2-4.
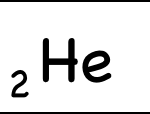
Example: Carbon-14
Carbon-14 is a radioisotope of carbon commonly used for carbon dating of historical artifacts. It is written as:
- Proton = 6: As long as it’s carbon it has six protons.
- Electrons = 6: This atom is also electrically balanced so it also needs six electrons.
- Neutrons = 8 : With an atomic mass of 14, when we subtract 6 protons, the number of neutrons should be 8 (14 – 6 = 8).
The only difference between carbon-12 and carbon-14 is that the latter has two more neutrons. So here are two isotopes of carbon.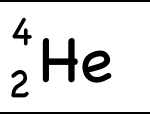
Example: Helium-4
Example: Sodium-23
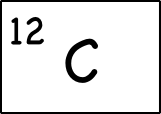
Last, Wallx.net sent you details about the topic “Drawing Atoms❤️️”.Hope with useful information that the article “Drawing Atoms” It will help readers to be more interested in “Drawing Atoms [ ❤️️❤️️ ]”.
Posts “Drawing Atoms” posted by on 2021-11-04 06:01:23. Thank you for reading the article at wallx.net
 The atomic number is written as a sub-symbol to the left of the element symbol.The atomic number is the number of protons. Since they’ve memorized the elements in order, they should be able to figure this out for themselves – but they can also do a quick lookup on the periodic table or look at the element symbol where the atomic number is sometimes written in bottom left .
The atomic number is written as a sub-symbol to the left of the element symbol.The atomic number is the number of protons. Since they’ve memorized the elements in order, they should be able to figure this out for themselves – but they can also do a quick lookup on the periodic table or look at the element symbol where the atomic number is sometimes written in bottom left . Atomic mass (4) is written as the symbol on the left side of the element symbol. Atomic mass is the sum of the number of protons (2) and the number of neutrons (2). The small atoms we are looking at tend to have the same number of neutrons as protons, but not necessarily. So how do you know how many neutrons there are? You have to ask, or look at the atomic mass number, which is usually written on the top left of the atom. Since atomic mass is the sum of the number of protons and neutrons, if you know the atomic mass and the number of protons you can easily find out the number of neutrons. (Note that electrons do not contribute to the mass of an atom because their mass is much smaller than that of neutrons and protons.
Atomic mass (4) is written as the symbol on the left side of the element symbol. Atomic mass is the sum of the number of protons (2) and the number of neutrons (2). The small atoms we are looking at tend to have the same number of neutrons as protons, but not necessarily. So how do you know how many neutrons there are? You have to ask, or look at the atomic mass number, which is usually written on the top left of the atom. Since atomic mass is the sum of the number of protons and neutrons, if you know the atomic mass and the number of protons you can easily find out the number of neutrons. (Note that electrons do not contribute to the mass of an atom because their mass is much smaller than that of neutrons and protons. This oxygen atom has 8 electrons in two shells.Electronic case: Electrons orbit the nucleus in a series of shells. Each shell can hold a certain maximum number of electrons (2 for the first shell; 8 for the second shell; and 8 for the third). And to draw atoms, you fill the inner shells first and then move on to the outer shells.
This oxygen atom has 8 electrons in two shells.Electronic case: Electrons orbit the nucleus in a series of shells. Each shell can hold a certain maximum number of electrons (2 for the first shell; 8 for the second shell; and 8 for the third). And to draw atoms, you fill the inner shells first and then move on to the outer shells.