Why Are More Substituted Alkenes More Stable
Alkene Stability (And Instability) What factors affect alkene stability? If you’ve studied elimination reactions, no doubt you’ve learned about Zaitsev’s Rule – about how elimination reactions generally favor the “more substituted” alkene.In this post we explore how increasing substitution at carbon increases the stability of alkenes, as well as the effects of conjugation and strain.Reading: why are more substituted alkenes more stableTable of Contents
1. Heat Of Hydrogenation As A Measure Of Alkene Stability
Contents
We might not spend as much discussing thermodynamics in here organic chemistry as you did in general chemistry, but that doesn’t mean the concepts have just gone away!One area where we’ve previously seen the usefulness of thermodynamic data is the use of heat of combustion data to quantify ring strain. [See: Cycloalkanes – How To Calculate Ring Strain]. The heat of combustion for cyclopropane works out to about 166 kcal/mol per CH2 compared to the heat of combustion for unstrained cyclohexane [157 kcal/mol per CH2]. That “extra” heat of combustion seen in cyclopropane is attributed to the instability arising from the strain of bent C-C bonds far away from their ideal angle of 109.5°. That’s angle strain.Another area of organic chemistry where thermodynamic studies are useful in the stability of alkenes.Back in 1935, Prof. Kiasatakowsky and co-workers at Harvard published a method for measuring the heat of hydrogenation of ethylene (aka “ethene”) as it was passed over a finely divided metal catalyst containing adsorbed hydrogen. [Note 1] Because hydrogenating a molecule is considerably more gentle than, say, BURNING it, the method tends to be more sensitive for determining subtle differences in enthalpies.In a hydrogenation reaction, a C-C bond is broken, and two new C-H bonds are formed.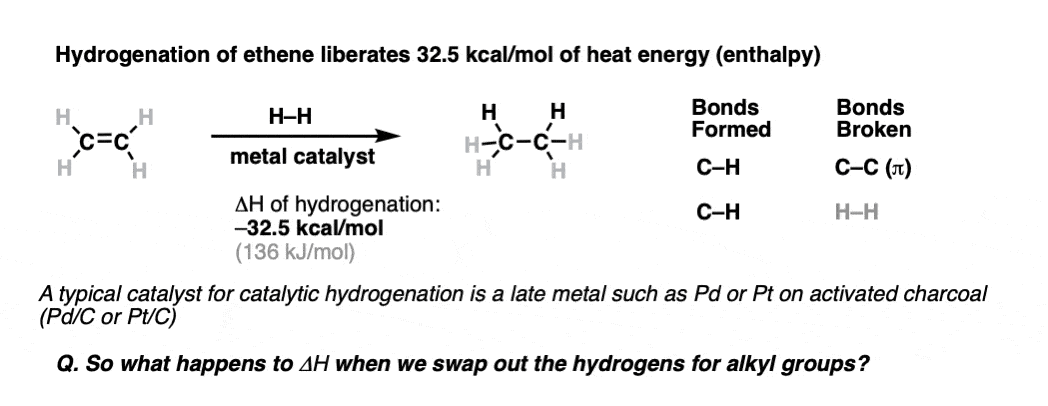
2. Stability of Alkenes Increases With Increasing Substitution
Well, as you might imagine from someone who had invented a new technique, Kiastakowsky went to town on this, investigating the heat of hydrogenation of a huge variety of alkenes. [Note 3] In the following decades, even more data has been accumulated, which is easily obtainable (with references) from the NIST Chemistry Web Book.For our purposes, there are six substitution patterns on an alkene (seven if you count ethene).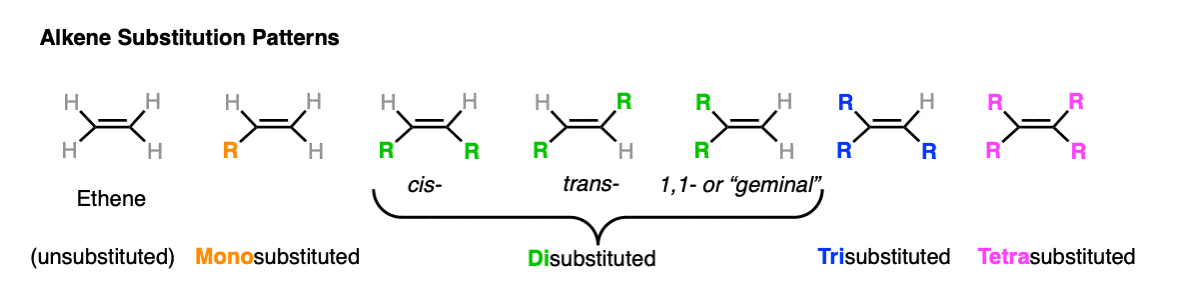
3. Heats Of Hydrogenation For Some Monosubstituted Alkenes
Just for fun, let’s look at a series of mono-substituted alkenes. Nothing weird here, we’ll just go from propene up to hex-1-ene.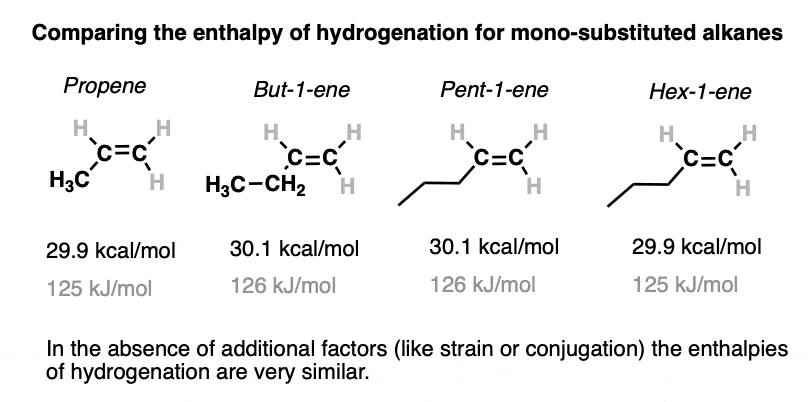
4. The Relative Stability of cis- and trans- Alkenes
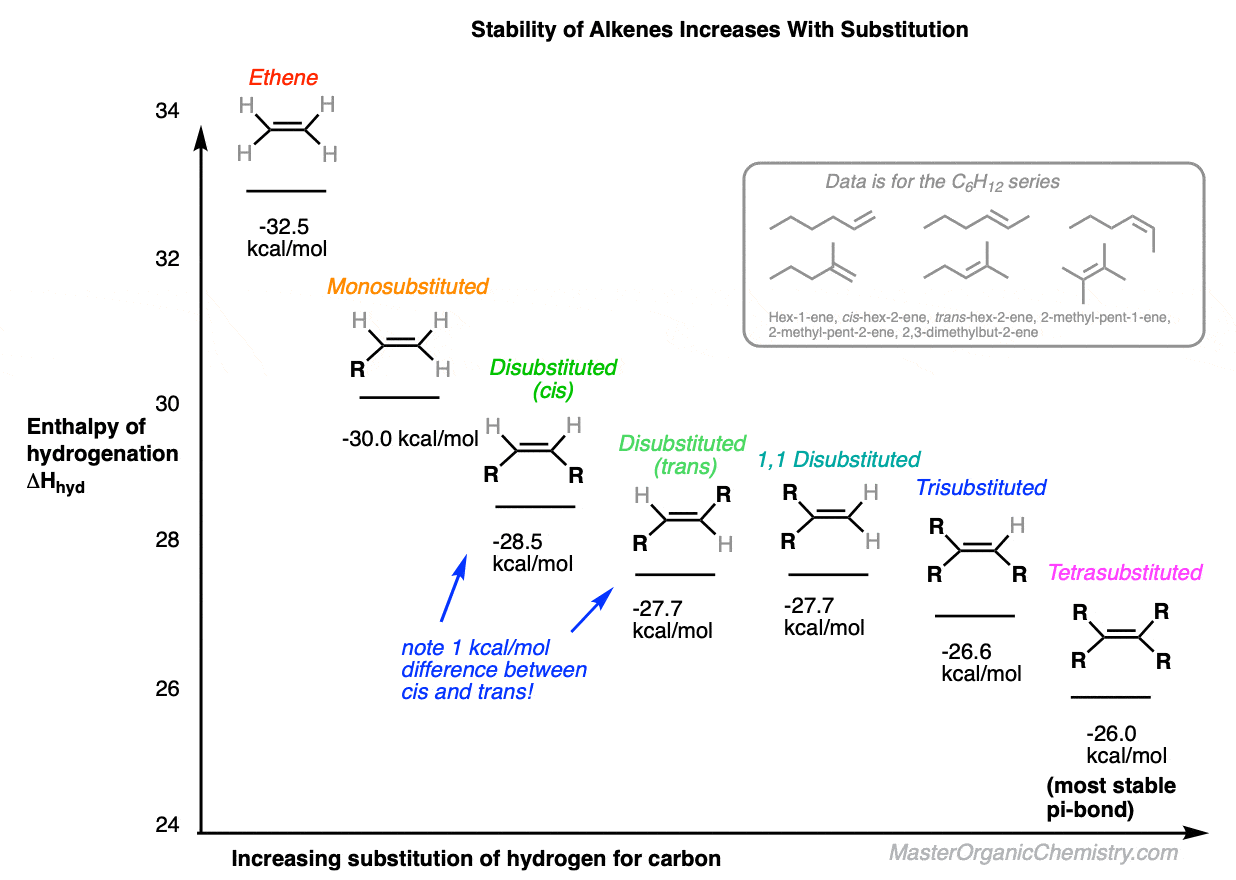
So what about disubstituted alkenes? There are three types (cis, trans, and 1,1-disubstituted) but let’s just concern ourselves with cis and trans here.We all know by now that cis and trans alkenes should differ a little bit in stability because in a cis alkene the groups are held closer together (more strain!) and in a trans-alkene they are further apart. [For a good time, amaze your instructor and call it by it’s proper name: 1,2-strain]Heat of hydrogenation data actually allows us to quantify the difference in stability between cis and trans alkenes.For instance, compare cis- and trans- but-2-ene, or cis- and trans hex-2-ene. The difference in stability is about 1 kcal/mol, rounding up generously.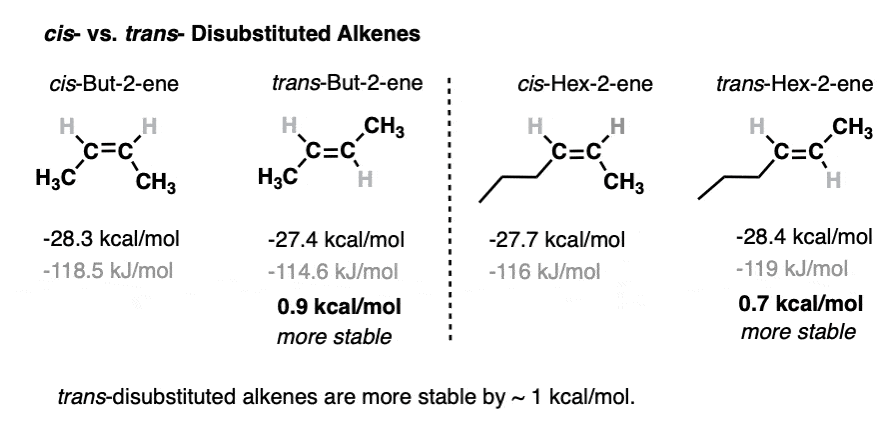
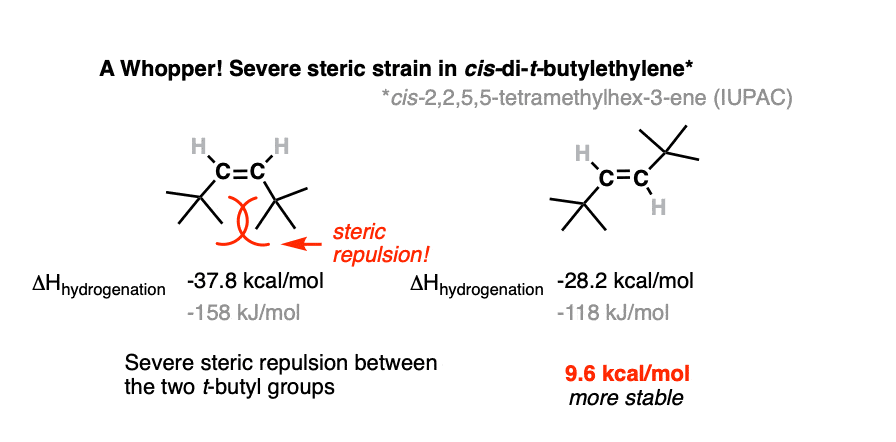
5. Alkenes Stabilized By Conjugation: Resonance Energy
The stability of alkenes is also affected by conjugation. This is a really a topic for another chapter [specifically, see Conjugation and Resonance] where we talk about pi systems, but the bottom line is that the p-orbitals in adjacent pi-bonds can clump together forming larger “pi-systems”, which provides more “room” for electrons to roam, lowering their energy. [Note 5]Heat of hydrogenation numbers allow us to quantify the effect of resonance stabilization. How so?Take but-1-ene. As we saw above the heat of hydrogenation is about 30.1 kcal/mol.Add a double bond, and you might expect the heat of hydrogenation to double as well. But it doesn’t! It’s actually a little bit less. [56.6 kcal/mol] . The difference (that extra 3.6 kcal/mol of additional stabilization) is called “resonance energy“.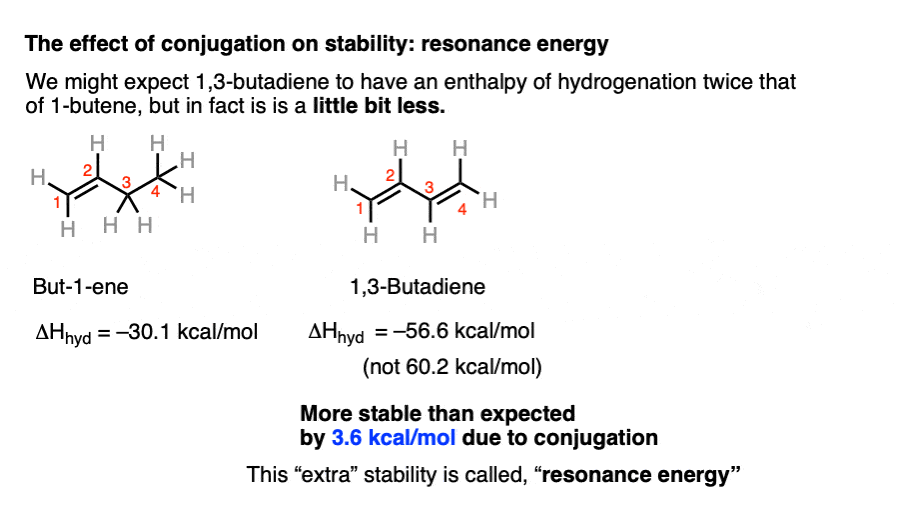
6. Summary: Stability of Alkenes
Read more: why are gaming laptops so expensive | Top Q&AThree key factors affect the stability of alkenes, and the influence of these factors can be measured through the enthalpy of hydrogenation.
- One important factor is the substitution pattern. As C-H bonds are replaced by C-C bonds, the stability of the alkene gradually increases in the order mono (least stable) < di < tri < tetrasubstituted (most stable).
- When hydrogenation liberates more energy than expected given the substitution pattern, that’s likely a sign of strain. This is exemplified in the difference in enthalpy of hydrogenation between cis- and trans- alkenes, where the trans- alkene is more stable by about 1 kcal/mol.
- When hydrogenation liberates less energy than expected given the substitution pattern, that’s a sign that some extra factor is stabilizing the molecule. Among commonly encountered factors, conjugation ranks high. The difference in energy between the “expected” heat of hydrogenation and the measured heat of hydrogenation is called the resonance energy. The conjugation of one pi bond with an additional pi bond is “worth” about 2-3 kcal/mol.
The increasing stability of alkenes with increasing substitution not only comes up in Zaitsev’s Rule, but also later in the course when you study Thermodynamic and Kinetic Control.
Notes
[1] It was a copper catalyst, after a lot of trial and error. The advantage of measuring the heat of hydrogenation over the heat of combustion is that it is a more sensitive technique for measuring small energies.[2] This number was first measured in 1935, remeasured in 1951, and so far as I am aware, has not been updated. See the entry in the NIST Chembook for ethylene.[3] Standard heats of hydrogenation have been pulled from the NIST Chembook.[4] Actually 82:18 at 298 K. From delta G = -RT ln K, using delta G of 1000 cal, T = 298 K, R = 1.987 cal / mol•K .[5] If you think of electrons as waves, a larger pi-system allows for longer wavelengths, and since energy is inversely proportional to wavelength, this means a lower overall energy of the electron.And a big thank you to The Kraken for his steady hands in the stability GIF.
Appendix 1: Why Does Increasing Substitution Increase Stability?
So why does increasing substitution at the alkene increase its stability? This is not an easy question to answer to an introductory audience in a few sentences, and given the time constraints of a typical course the answer you will generally get from an instructor will range from “it’s complicated” to “hyperconjugation” to “orbital mixing”. Very rarely you might get an MO diagram.The unifying principle here is that full orbitals – even those from single bonds – can donate into empty (even antibonding) orbitals, and that this interaction is stabilizing.In ethene (below left) all of the C-H bonds are in the plane of the alkene, and none can overlap with the pi bond.When a methyl group is added, say, in propene, one of the C-H bonds can now align with the pi-system of the alkene. The pair of electrons from the C-H bond can then donate into the empty pi* orbital.This can be visualized through “no-bond resonance”, below right, where a “resonance” form is shown with a broken C-H bond and a new C-C pi bond. [The quotation marks are to differentiate it from our traditional view of resonance where only pi-bonds are allowed to form and break].This mixing results in a stabilization of the molecule. . Although CH3 is in rapid rotation, at any given moment at least one of the C-H bonds will have the proper geometry to allow overlap with the pi system. 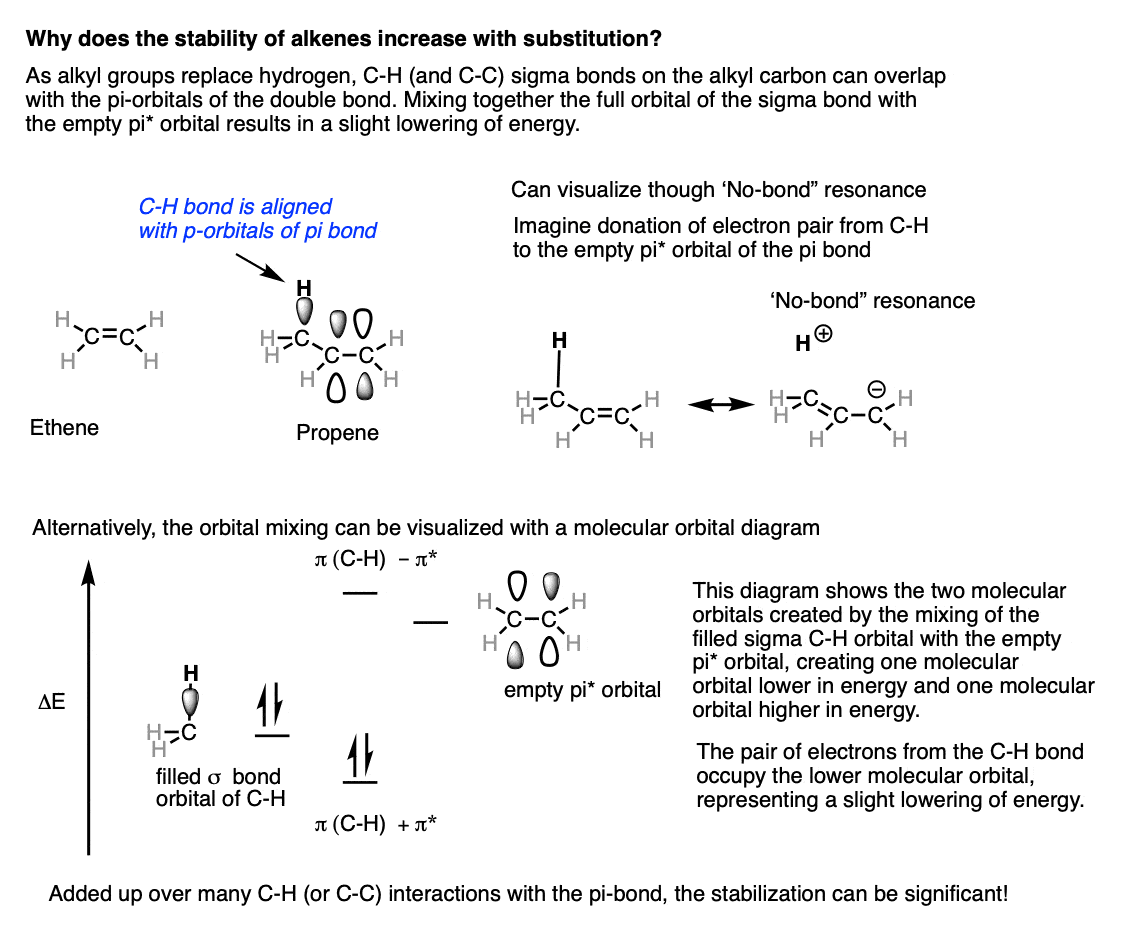
Appendix 2: trans-Cycloalkenes
99% of people reading this will never use this so it is going down in the footnotes.In the vast majority of molecules you will encounter, the double bonds in rings are cis. Why? The most vivid answer is provided by trying to make them with a model kit.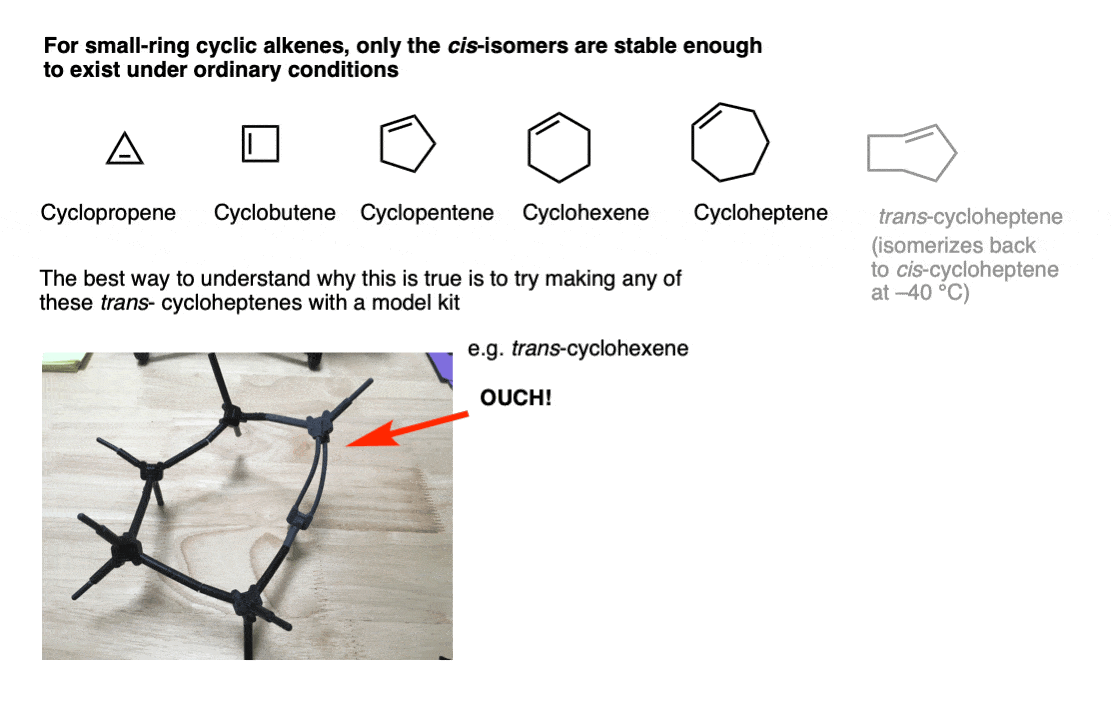
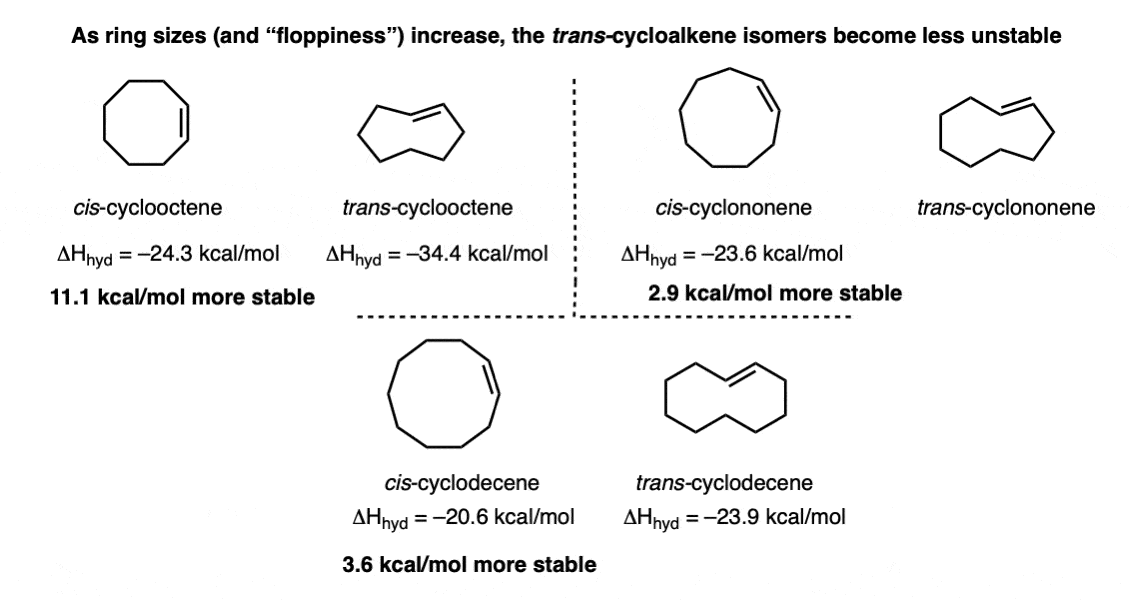
Quiz Yourself!
(Advanced) References and Further Reading
All heat of hydrogenation values cited here were obtained from the NIST Chemistry Web Book. Searching by CAS number never fails. Selected original references below.Read more: Angry Ex? Here’s What To Do…
Last, Wallx.net sent you details about the topic “Why Are More Substituted Alkenes More Stable❤️️”.Hope with useful information that the article “Why Are More Substituted Alkenes More Stable” It will help readers to be more interested in “Why Are More Substituted Alkenes More Stable [ ❤️️❤️️ ]”.
Posts “Why Are More Substituted Alkenes More Stable” posted by on 2021-09-08 05:46:40. Thank you for reading the article at wallx.net