Is BF3 Polar or Nonpolar?
BF3, also known as Boron Trifluoride, is an inorganic chemical compound, is a colorless gas, with a pungent odor, students are often confused about the polarity or nonpolarity of BF3 (Boron Trifluoride) due to the presence of three Fluorine atoms has a very large electronegativity value when compared to the Bo atom. Read: why is bf3 non-polar So, is BF3 polar or non-polar? BF3 (Boron Trifluoride) is Non-polar because of its highly symmetric shape. It has a triangular plane geometry that removes the dipole moments of the three BF bonds making the resulting compound’s resulting dipole moment zero (Zero).Let’s try to understand this in detail. To answer our question, we will analyze the different factors, responsible for polarization, for BF3.
Molecular Structure of BF3 (Boron Trifluoride)
Contents
The molecule BF3 (Boron Trifluoride) has 1 B atom (Bo, atomic number: 5) and 3 F atoms (Fluorine, atomic number: 9). The valence of B (Boron) is 3 and that of F (Flo) is 7, so the Lewis structure of BF3 can be drawn as shown in the figure:Read more: Why do cats sometimes sleep in their litter box | Top Q & AWe can see that each F(Flo) atom has 3 unique electron pairs, so the molecular structure is balanced and symmetrical. geometry with each FBF bond angle equal to 120 degrees, which again adds to the balance of the molecule making it highly symmetric, as shown: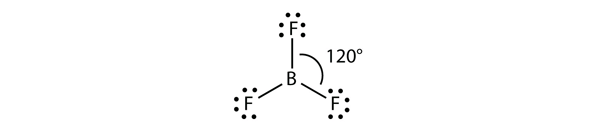
Electronegativity & Bond Polarity of BF3 (Boron Trifluoride)
In the BF3 molecule (Boron Trifluoride) there are three BF bonds, as clearly shown in the Lewis diagram above. The electronegativity of B (Boron) is 2.04 and that of F (Flo) is 3.98 (peak) on the Pauling scale which means that F (Flo) will pull the shared electrons towards itself and thus will get a partial negative charge (δ-) and B (Boron) will have a partial positive charge (δ +). To determine if a bond is polar, we must Find the difference in electronegativity of two atoms sharing a bond. The electronegativity difference of B and F by 1.94 (3.98 – 2.04 = 1.94) is greater than 0.5, i.e. each BF bond in the BF3 (Boron Trifluoride) molecule is polar. That means the electrons are not shared equally by the two atoms (B and F) instead they are pulled towards F (Flo). Bond polarity does not always lead to the overall polarity of the molecule.Read more: Why do cats sometimes sleep in their litter boxes | Top Q&A
BF3 (Boron Trifluoride) Dipole Moment
The three bonds of BF in BF3 (Boron Trifluoride) are polar (as discussed above) and thus they have a bonding dipole moment as shown below: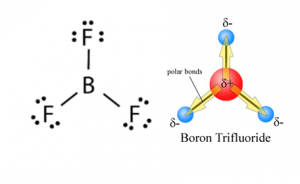
Molecular Structure & Geometry
The polarity of a molecule is highly dependent on its structure and geometry. Since the bond angle between the bonds determines if the resulting dipole moment of the overall molecule is zero (Zero). The Lewis structure of the molecule gives us an overview of all the lone electron pairs present in the molecule. have a single pair). Molecules with high symmetry are Nonpolar (like BF3). sometimes sleep in their litter tray | Top Q&A
Electronegativity
Electronegativity is denoted by the symbol χ (the Greek letter Chi). It can be defined as the ability or force with which the atom of an element pulls the shared electrons of a bond towards itself making the sharing unequal. bonding pairs with respect to itself. It is determined by two factors, the first is the atomic number and the second is the distance between an atom’s valence electrons and its positively charged nucleus. was given by Linus Pauling. According to the element fluorine has the greatest electronegativity of 3.98.Read more: Why do cats sometimes sleep in their litter trays | Top Q&A
Bond polarity
To know if a compound is polar or nonpolar, we must first have an idea of what polarity means. In this bond electron sharing takes place but this sharing is always unequal between the elements. partial positive charge (δ +). As a result, charge separation in the bond takes place and polarization of the bond develops. For a bond to be polar, the electronegativity difference between the two atoms must be greater than or equal to 0.5. However, the polarity of the bond alone does not make the molecule polar. Read more: Why do cats sometimes sleep in their litter box | Top Q&A
Dipole moment
When a bond is polarized i.e. charge separation occurs, the dipole moment of the bond is formed. It is a measure of the polarity of the bond formed by two atoms. -Since dipole moment is a vector quantity i.e. it has both magnitude and direction, so it can also be zero (Zero) when two oppositely bound dipoles cancel each other out due to symmetric geometry of the molecule, as in BF3. measured in Debye Units, denoted by ‘D’. 1 D = 3.33564 × 10-30 Cm, where C is Coulomb and m stands for meter. To calculate the dipole moment we must know the shape and structure of the molecule. Mathematically, the dipole moment of a bond can be calculated using the following formula:Dipole moment (µ) = Charge (Q) * separation distance (r)A molecule can have all its bonds polar but it can be nonpolar (like BF3) it happens because the cancellation of the dipole moment of all the bonds makes the resulting dipole moment equals 0 (None). Now, with the concept clear in our mind, we can clearly understand how the BF3 molecule is nonpolar despite the presence of F(Flo), a highly electronegative element Read More : Why do cats sometimes sleep in their litter tray | Top Q&A
Properties of BF3
Boron Trifluoride is toxic in the gaseous state but readily soluble in cold water due to its high solubility and produces highly corrosive hydrofluoric acid, which can corrode metals including stainless steel. Its solubility in cold water is 106%. Vapors of BF3 are heavier than air and prolonged exposure of BF3 containers to heat or fire may cause rupture or fire. Stable in dry air environment. box | Top Q&A
Uses of BF3 (Boron Trifluoride)
BF3 (Boron Trifluoride) has a wide range of applications in various industries, used as adhesives and sealants, adsorbents and absorbents, fuels and fuel additives, oxidizers/ reducing agent, binder for industrial production, plastic industry, drug manufacturing, etc. (Source: topqa.info/chemical-data-reporting) The paper industry also uses BF3 (Boron Trifluoride) to produce pulp. It is also used as engine lubricant, brake fuel, oil, etc. In addition, it is useful in the production of oil, crude oil, crude oil, refined oil products, fuel oil, oil. drilling, etc. of BF3 (Boron Trifluoride) occurs in organic synthesis as a catalyst for many reactions useful in industrial production. Some of them are mentioned below:
- Friedel-Crafts . alkylation reaction
- The cleavage of ethers into alcohols
- Esterification reaction
- Nitrification and sulphonation of aromatic compounds (Source: Brotherton RJ et al.; Ullmann’s Encyclopedia of Industrial Chemistry 7th edition (1999-2012))
By now you should have understood the concept behind polarity and nonpolarity of a molecule, including the specific reasons for BF3 (Boron Trifluoride) to make it nonpolar. Please leave your questions in the comments section below. We will get back to you as soon as possible.Read more: why does my windows media player keep crashing | Top Q&A
Last, Wallx.net sent you details about the topic “Is BF3 Polar or Nonpolar?❤️️”.Hope with useful information that the article “Is BF3 Polar or Nonpolar?” It will help readers to be more interested in “Is BF3 Polar or Nonpolar? [ ❤️️❤️️ ]”.
Posts “Is BF3 Polar or Nonpolar?” posted by on 2021-08-21 16:32:10. Thank you for reading the article at wallx.net