Hybrid Orbitals
Video Why are orbitals hybrid?
sp . hybridization
sp Hybridization can explain the linear structure of the molecule. In which, the 2s orbital and one of the 2p orbitals hybridize to form two sp orbitals, each consisting of 50% s and 50% p characters. The anterior lobes face away from each other and form a straight line leaving a 180° angle between the two orbitals. This formation minimizes electron repulsion. Because only one p orbital is used, we are left with two unchanged 2p orbitals that the atom can use. These p orbitals are perpendicular to each other and form a line formed by two sp orbitals.Energy change occurs during hybridizationRead: why are orbitals hybrid?Read more: Moms turning brown: What does this mean for your plant? Figure 1: Notice how the energy of the electrons decreases during hybridization. link, resulting in a triple bond. This only happens when two atoms, such as two carbons, both have two p orbitals that each contain one electron. An sp hybrid orbital results when an s orbital combines with a p orbital (Figure 2). We will get two sp hybrid orbitals since we started with two orbitals (s and p). sp hybridization produces a pair of sp hybrid orbitals with opposite directions. These hybrid orbitals result in higher electron density in the binding region for the sigma bond towards the left side of the atom and for the other sigma bond towards the right. In addition, sp hybridization provides a linear geometry with a bond angle of 180°. Example: Sp hybridization in Magnesium Hydride In magnesium hydride, the 3s orbital and one of the 3p orbitals from magnesium hybridize to form two sp orbitals. The two frontal lobes of the sp orbital point away from each other forming a straight line leading to a linear structure. These two sp orbitals are bonded to two 1s orbitals of two hydrogen atoms through sp-s orbital overlap. Read more: Why is wendy so annoying in ozarkHybridization 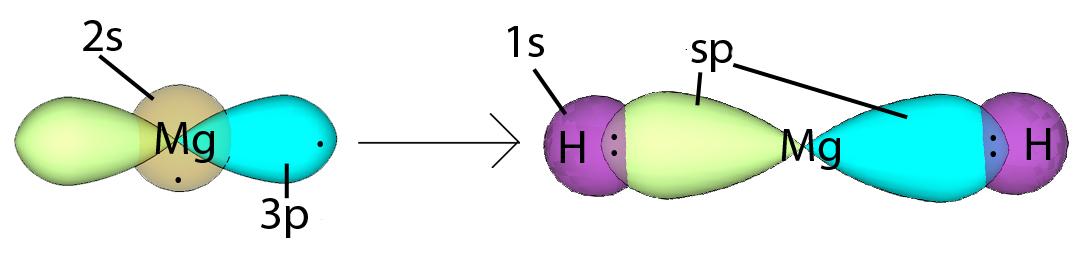 Example: sp hybridization in ethyne The hybridization in ethyne is similar to the hybridization in magnesium hydride. For each carbon, the 2s orbital hybridizes with one of the 2p orbitals to form two sp hybrid orbitals. The frontal lobes of these orbitals face away from each other to form a straight line. The first bond consists of sp-sp orbital overlap between the two atoms. Two other bonds include the overlapping of the s-sp orbitals between the sp hybridized orbitals of the carbons and the 1s orbitals of the hydrogens. This leaves us with two p orbitals each with a single carbon in them. Does this allow the formation of two? linked through pp interspersed orbitals. The linear shape, or 180° angle, formed due to the minimized electron repulsion is greatest at this position.Hybridization
Example: sp hybridization in ethyne The hybridization in ethyne is similar to the hybridization in magnesium hydride. For each carbon, the 2s orbital hybridizes with one of the 2p orbitals to form two sp hybrid orbitals. The frontal lobes of these orbitals face away from each other to form a straight line. The first bond consists of sp-sp orbital overlap between the two atoms. Two other bonds include the overlapping of the s-sp orbitals between the sp hybridized orbitals of the carbons and the 1s orbitals of the hydrogens. This leaves us with two p orbitals each with a single carbon in them. Does this allow the formation of two? linked through pp interspersed orbitals. The linear shape, or 180° angle, formed due to the minimized electron repulsion is greatest at this position.Hybridization 
 Read more: why daft punk wears helmets | Top Q&A
Read more: why daft punk wears helmets | Top Q&A
Last, Wallx.net sent you details about the topic “Hybrid Orbitals❤️️”.Hope with useful information that the article “Hybrid Orbitals” It will help readers to be more interested in “Hybrid Orbitals [ ❤️️❤️️ ]”.
Posts “Hybrid Orbitals” posted by on 2021-08-15 10:38:10. Thank you for reading the article at wallx.net