2.3D: Separation Theory
General theory
TLC is an excellent analytical tool for separating mixtures in a sample. In this section the separations are discussed in detail and extended to the general discussion of Section 2.1.B. In all forms of chromatography, the samples are in equilibrium between the stationary and mobile phases. In most applications of TLC, the stationary phase is a silica or alumina adsorbent and the mobile phase is an organic solvent or a mixture of solvents (“elution“) Pick up the plate (equation 3). Read: why is ethyl acetate polar[ce{X}_text{(silica/alumina)} rightleftharpoons ce{X}_text{(solvent)} label{3}]Silica gel (shown in Figure 2.16) is composed of a network of silicon-oxygen bonds, with (ce{OH}) bonds on its surface, as well as a layer of water molecules. Silica gel (left (ce {SiO_2} cdot x ce {H_2O} right)) is used in this discussion, but is structurally similar to alumina (left (ce {Al_2O_3} cdot x ce {H_2O} right) ). This highly polar stationary phase is coupled to a relatively nonpolar mobile phase (solvent or organic solution), which is referred to as the “normal phase” TLC. Although this is the most common form of TLC (and what will be focused on in this section), “reverse phase” TLC (with a non-polar stationary phase and a polar mobile phase) is sometimes used. surface of silica gel through intermolecular forces (IMF). In this case, acetophenone can hydrogen bond (IMF shown in Figure 2.16a) to the silica surface through its oxygen atom. As the eluate flows through the sample (Figure 2.16b), an equilibrium is established between the sample adsorbed on the stationary phase and dissolved in the mobile phase. Once in the mobile phase, the compound moves onto the plate with the liquid stream (Figure 2.16c) so that the reading permeable to the stationary phase continues onto the plate. The result (R_f) of the compound depends on the amount of time spent in the stationary and mobile phases.Figure 2.16: Structural diagram of compounds bound to silica-coated TLC sheet (right figure). In fact, the silica layer is much thicker than pictured ((0.25: letter {mm}) of the powder), and the surface is more porous and uneven than implied. a) Acetophenone is detected on the baseline of the TLC plate, b) The eluent crawls onto the TLC plate, c) Acetophenone in the mobile phase after breaking its IMF with the silica surface. Further reading: why do the guys disappear and then reappear | Q&A The balance distribution between the two periods depends on several factors:
The degree of attraction of a compound to the stationary and mobile phases leads to the same conclusion:
- The stronger the IMF is with the stationary phase (usually the more polar functional groups per compound), the lower the time the compound stays in the stationary state (right arrow) (R_f).
- The more polar functional groups a compound has, the less likely it is that the compound will be attracted to the less polar eluent, and the less time the compound has to be mobile (right arrow) (R_f).
Therefore, a compound with a lower (R_f) tends to have more polar functional groups than a compound with a higher (R_f) (summarized in figure 2.17) .Read more: why do women pierce their tongue | Top Q&A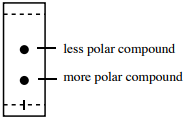
Last, Wallx.net sent you details about the topic “2.3D: Separation Theory❤️️”.Hope with useful information that the article “2.3D: Separation Theory” It will help readers to be more interested in “2.3D: Separation Theory [ ❤️️❤️️ ]”.
Posts “2.3D: Separation Theory” posted by on 2021-08-29 08:37:05. Thank you for reading the article at wallx.net