True Solution | Top Q&A
Video What is the Real Solution?
What is the real solution?
Contents
A correct solution is a solution that includes all particles of the correct composition and has been properly dissolved. As a result, a solution is called a true solution, a true solution is a homogeneous mixture with consistent properties. Filtration cannot separate the solute from the solution in the true solution. The particle size of the solute is equivalent to the size of the solvent and the solvent and solute together move through the filter paper. (solute) in solvents less than 10-9 m or 1 nm. The correct solution is e.g. a solution of simple sugars in water.
True Solution’s Attributes
Read more: What is a football cover A true solution is a mixture of a solute and a solvent that is homogeneous. Filtration cannot separate the solute from the solution in the true solution. The particle size of the solute is equivalent to the size of the solvent, the solvent and the solute moving together through the filter paper. The solute particles are smaller than 1 nm (1 nm = 10-9m). The elements do not scatter light and do not exhibit the Tyndall effect. The filtration will not be able to separate the particles. The result is safe (still homogeneous). The solution is visible. In a true solution, the solute particles do not settle. Light is not scattered in a real solution. A real solution is transparency and clarity.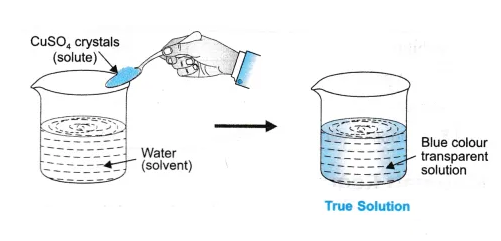
The difference between True solutions and glue solutions
Read more: LCM of 24 and 36 | Q&AIn top chemistry terms, A solution is classified as a mixture of two or more substances, where the solvent is a liquid and the solute is a liquid, solid or gas. There are several types of solutions, each with its own set of characteristics, but they can be divided into: True Solution and Glue Solution. The comparison chart is given below. Comparative basis Correct solution Glue solution Definition A real solution is a homogeneous mixture of two or more substances in which the particle size of the dissolved material (solute) in the solvent is less than 10-9 m or 1 nm. A colloid is a mixture in which one material is suspended in another by microscopically dispersed insoluble particles. Examples Sugar solution in water. Starch is soluble in water. Nature of Solution Colloids and suspensions are heterogeneous mixtures of two or more substances, while true solutions are homogeneous mixtures. Another difference between these three solutions is that True is transparent, while Colloidal is translucent and Suspension is non-transparent. A colloidal solution, also known as a colloidal suspension, is a mixture in which substances are dissolved in a liquid in a normal pattern. A colloid is a very small particle that is evenly distributed in another substance. In essence, they are not identical. Appearance A true solution is one that is both simple and transparent. Light is not scattered in real solution. Filtration cannot distinguish the components of a real solution. The Tyndall effect, which is the scattering of light by particles in colloids, causes some colloids to be translucent. Sediment Will, not sediment. There will be no deposition of particles or colloids. The visibility of True Solution particles is invisible to the naked eye. As light travels through colloidal solutions, it is scattered, with the scattering intensity being highest in a plane perpendicular to the direction of light. The beam is clearly visible. Brownian Motion Brownian motion is observed in real solution particles. Brown motion is observed in the colloidal solution particles.
Prepare the right solution
1. Correct solution of common salt:
Pour 100 mL of distilled water into a clean, dry beaker, then add the usual dry salt. Use a glass rod to stir. To form a correct solution, the salt is usually completely soluble.
2. The real solution of the road:
Pour 100 mL of distilled water into a clean, dry beaker, add a few sugar crystals, and stir with a glass rod. To form a true solution, sugar dissolves in water.
3. Correct solution of alum:
Pour 100 mL of distilled water into a clean, dry beaker, then add a pinch of alum powder and mix well with a glass rod. A correct solution is formed when alum is dissolved in water. Read more: what is the square root of 256 | Top Q&A
Last, Wallx.net sent you details about the topic “True Solution | Top Q&A❤️️”.Hope with useful information that the article “True Solution | Top Q&A” It will help readers to be more interested in “True Solution | Top Q&A [ ❤️️❤️️ ]”.
Posts “True Solution | Top Q&A” posted by on 2021-08-18 03:08:11. Thank you for reading the article at wallx.net

