Conjugation And Resonance In Organic Chemistry
Conjugation In Organic Chemistry: Definition, Examples, Exploration, and ConsequencesThis is the first in a series of posts that will eventually cover conjugation, pi systems, molecular orbital theory, dienes, 1,2- and 1,4- additions, the Diels Alder reaction and other pericyclic reactions. We’re going to start by reviewing the basics!Table of Contents
1. Revisiting the Pi Bond (and Pi bonding): “Side-On” Orbital Overlap Between Adjacent p-Orbitals
Contents
One of the first things you learn about alkenes is that rotation about the C-C pi (π) bond does not occur. For instance, at normal temperatures and pressures., trans-2-butene (shown below left) is never observed to spontaneously convert to cis-2-butene (right) . They’re separable compounds, with different melting and boiling points. You can buy each of them separately from Aldrich. This wouldn’t be possible if there was free rotation about the double bond.Digging deeper into this, we’ve seen that this is due to a phenomenon called “pi bonding” – a side-on overlap of two adjacent p orbitals, each containing an electron, which results in a preferred orientation where the p-orbitals “line up” next to each other, like soldiers. Due to the dumbbell-like geometry of the p-orbital, overlap isn’t possible when the two p-orbitals are at 90° to each other, which accounts for that “rotational barrier”.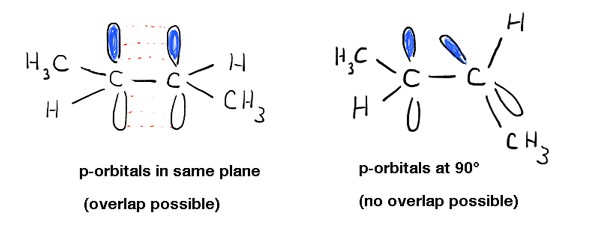
2. The Importance Of Orbital Overlap For Pi Bonding
A vivid illustration of the importance of orbital overlap is presented by a case where we might naively think a double bond “should” form – but does not. Bredt observed in 1924 that alkenes tend not to form on “bridgehead” positions, such as in the molecule at bottom left, an observation that came to be called “Bredt’s rule“.Why not? If you make the model, you’ll see that the geometry of the bicyclic ring forces those p orbitals to be oriented at right angles. There’s no overlap between the p orbitals. Therefore, it resembles a carbon with two adjacent radicals more than it does a real pi bond!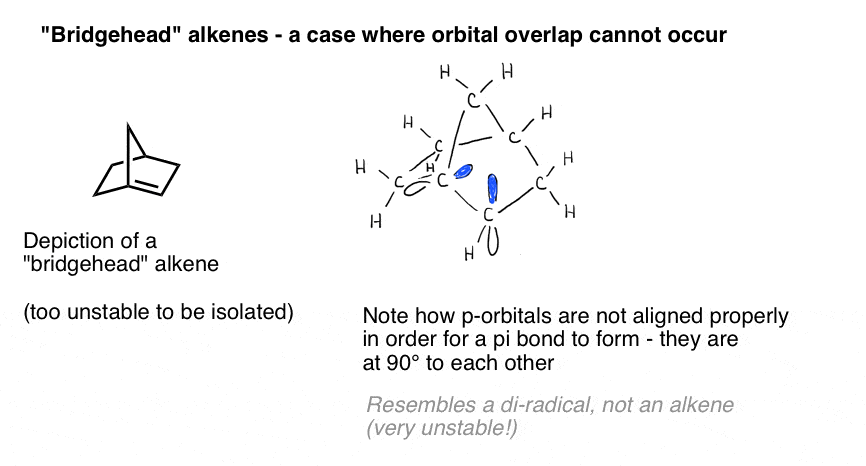
3. Beyond Pi Bonds: “Conjugation” Of 3 Or More p Orbitals
In first semester organic chemistry we learn that this overlap of p orbitals is not necessarily confined to two adjacent p orbitals. Overlap can extend beyond two p orbitals to include three, four, five, and even more consecutive p orbitals on consecutive atoms, building larger “pi-systems” (witness lycopene, for instance).We also see that definition of “p orbital” is somewhat flexible, and can include examples such as
- an empty p orbital (such as that in a carbocation, or the empty p orbital on boron)
- an orbital containing a lone pair (e.g. on nitrogen, oxygen, fluorine, etc.)
- p-orbitals of a pi bond [such as another alkene, C=O (carbonyl), etc.]
- a half-filled orbital (e.g. a radical)
We call this “building up” of p orbitals into larger “pi systems”, “conjugation”. In each of the middle molecules below, the alkene (pi bond) is conjugated with an adjacent p orbital.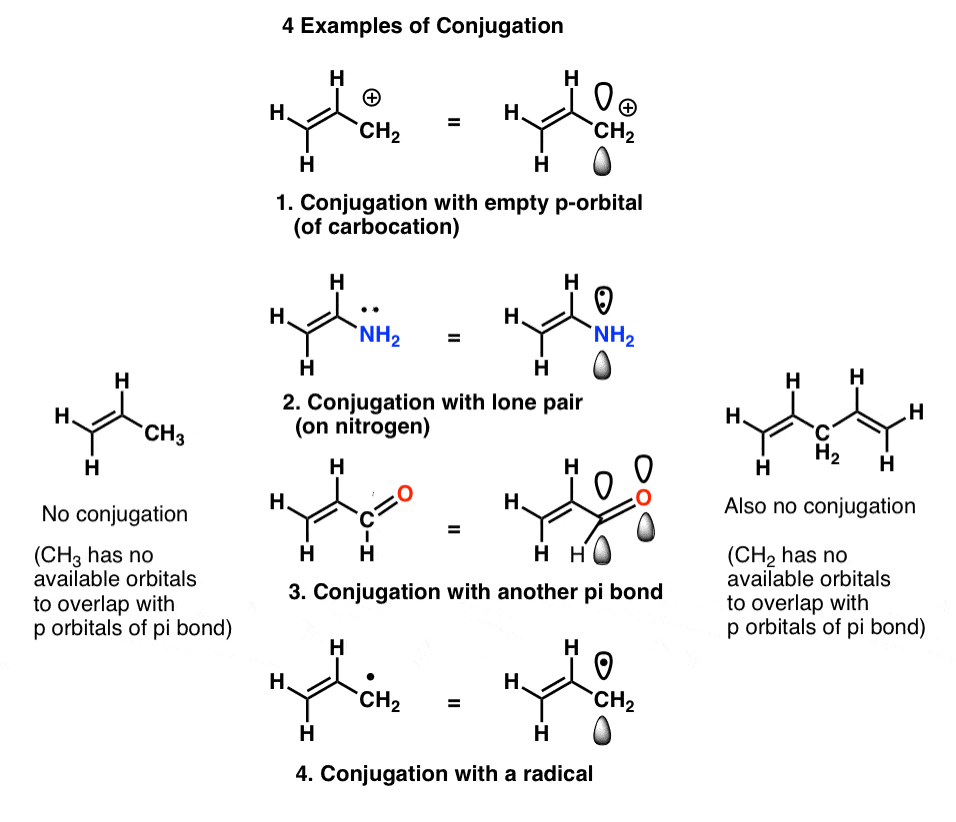
4. The Distinction Between Conjugation And Resonance
You might well ask: this just sounds like resonance. What’s the difference?Let’s take a second to distinguish conjugation and resonance.
- Conjugation is what we call it when 3 or more p orbitals join together into a larger “pi system”.
- These conjugated pi systems contain electrons, which we often call “pi electrons” to distinguish them from the electrons that comprise single bonds in the molecule.
- The different arrangements of electrons within that “pi system” are called resonance forms.
A rough analogy could go like this:
- Think of p orbitals as being a bit like “rooms” for electrons (maximum occupancy: 2)
- Joining several rooms together into a larger building is conjugation
- The different allowable arrangements of people (electrons) within that building are resonance forms.
The key requirement for conjugation is orbital overlap, which we’ll expand on in a bit.For now, let’s review some consequences of conjugation.
5. Consequences of Conjugation (1): Bond Lengths
As I just said, we’re more used to conjugation in the context of “resonance“, a concept we’ve covered before (and since this series is going into second-semester territory, is worth re-familiarizing yourself with)For instance, with the acetate ion (CH3CO2)- and the allyl cation (both shown below), we saw that there’s two different ways of arranging the pi electrons, which we call “resonance forms”.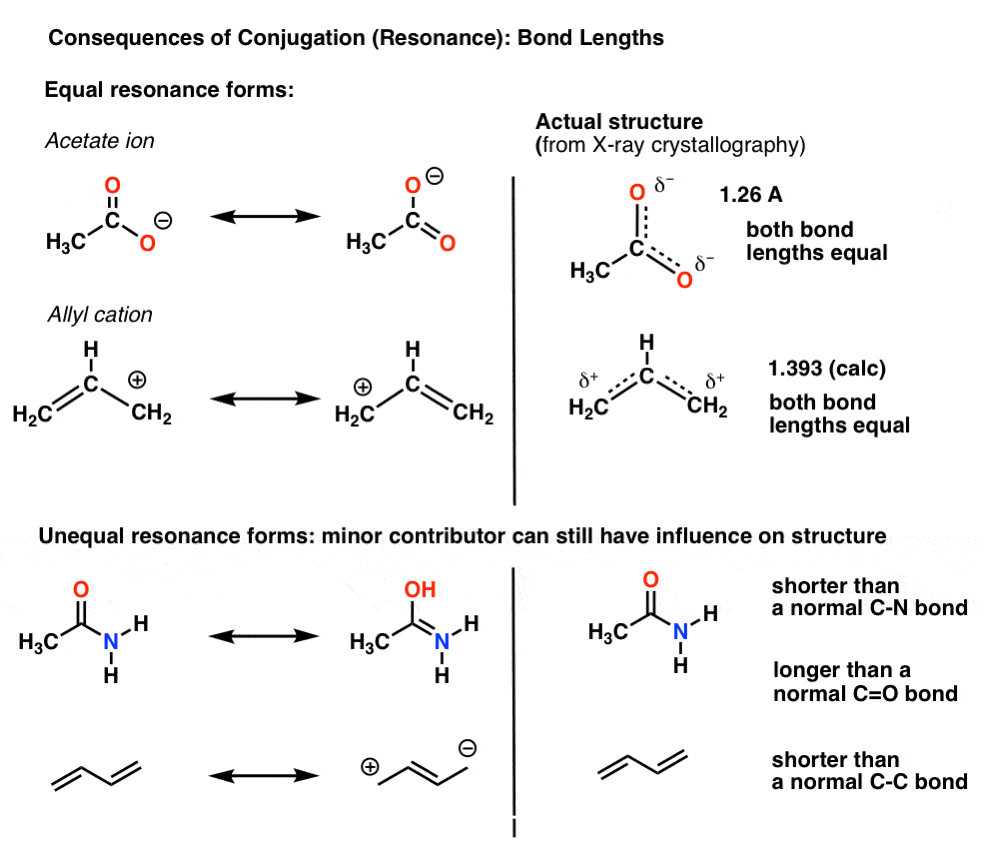
6. When Resonance Forms Are Not Identical, The Resonance Hybrid Will Be A “Weighted” Hybrid Of The Most Important Resonance Forms
In the acetate ion and the allyl cation the two important resonance forms are equivalent, so both end up contributing equally to the hybrid.A more common situation is found molecules like the ones below there’s a blending of unequal resonance forms. Some resonance forms are more important than others. [to go back to determining how to evaluate resonance forms, go to this series of posts. or these videos].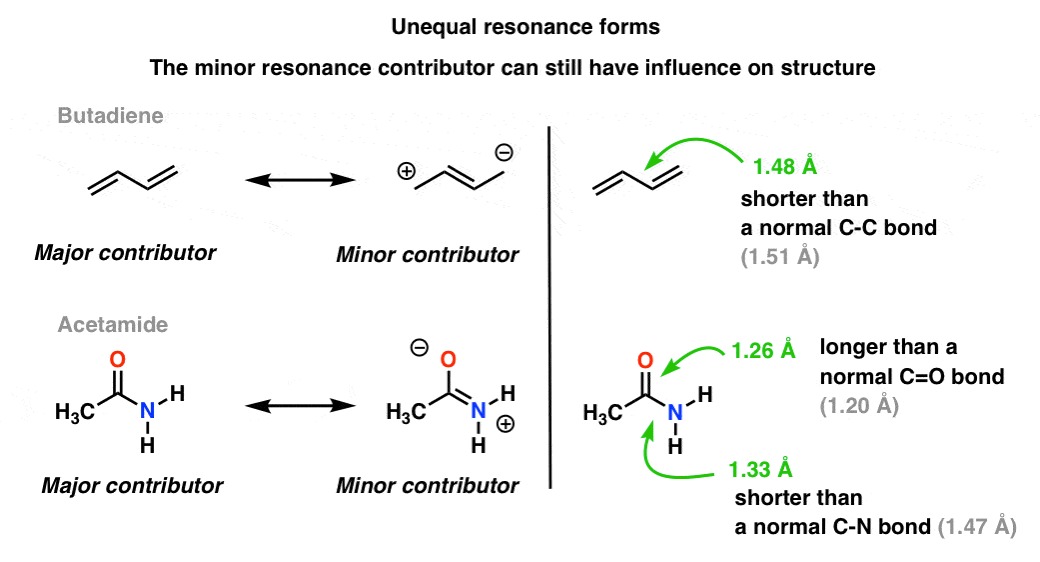
7. Consequences Of Conjugation (2): “Partial” Double Bonds
There’s an interesting consequence of that “partial double bond character” in the C-N bond. It has a “barrier to rotation” just like we’d expect from a “double bond”! The barrier to rotation in the C-N bond of amides is about 15-20 kcal/mol in peptide bonds (compare to about 2-3 kcal/mol for most C-C bonds).What this means is that the two conformations can still interconvert, but they do so relatively slowly at room temperature. In the molecule below (N-methyl acetamide) it’s possible to observe the s-cis conformer (both green methyl groups on the same side of the C-N bond) and the s-trans conformer (green methyl groups on opposite sides of the C-N bond) separately. [note] This usually isn’t possible for conformers unless you take the temperature down to 100 Kelvin or so!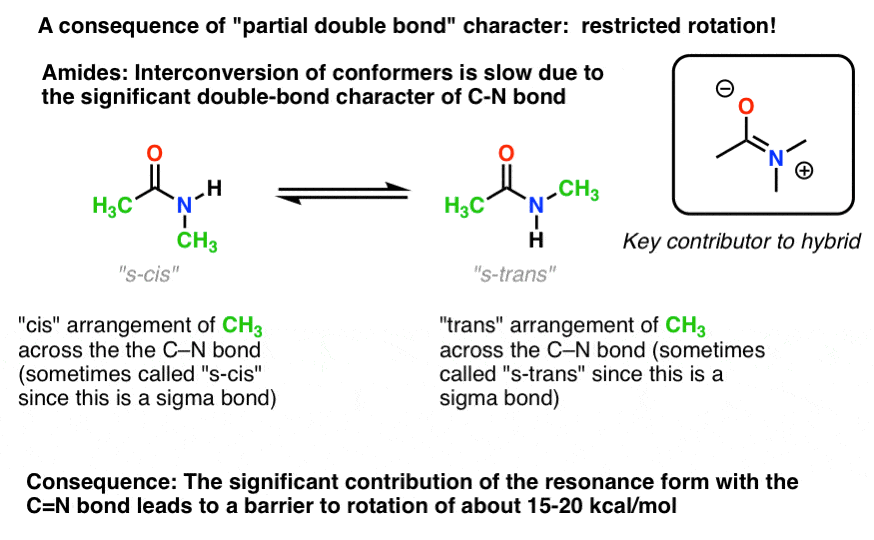
8. Consequences of Conjugation (3): The Reactivity Of A Conjugated System Is Often Revealed By Its “Second-Best” Resonance Form
We’ve shown the effect that conjugation (and by extension, resonance) has on bond lengths.Let’s take a closer look at its effect on electron density, which ultimately influences reactivity.For illustrative purposes, let’s continue to look at alkenes.The reactivity of an alkene can be modified dramatically through the attachment of various groups.Look at the “second best” resonance form when we attach a pi-donor such as N(CH3)2 to an alkene. This results in a build-up of negative charge (δ-) on terminal carbon of the alkene, with the result that this alkene (which we call an enamine) is an excellent nucleophile. To take just one prominent example, enamines react with alkyl halides (such as CH3I) and other electrophiles in a class of reactions sometimes referred to as Stork Enamine reactions after their discoverer, Gilbert Stork. Ordinary alkenes such as 2-butene (below) don’t work in this reaction.Attachment of a pi-acceptor such as C=O results in a build-up of positive charge (δ+) on the terminal carbon of the alkene, with the result that this species (which we call an α, β unsaturated aldehyde, Michael acceptor, or enone) is an excellent electrophile. α, β unsaturated carbonyls react with nucleophiles (such as CH3S-) and many other classes of nucleophiles in a general type of reaction we call conjugate additions or sometimes Michael reactions.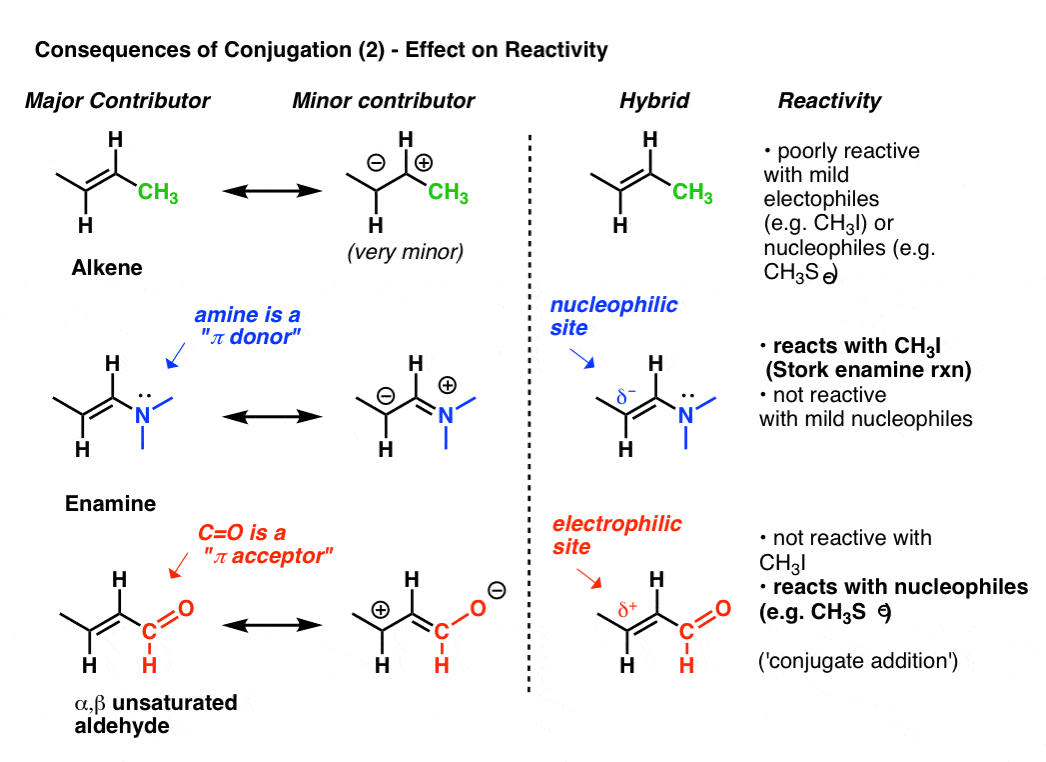
9. Orbital Overlap (All p-Orbitals In The Same Plane) Is Required For Conjugation (And Resonance)
So far we’ve seen that:
- overlap between p orbitals is necessary to form pi bonds
- some “single bonds” can have “pi bond character” due to contribution from a minor resonance form (such as amides, for example)
Here is the logical consequence of these two statements:
- In order for conjugation to exist, and therefore in order for resonance to occur, all the p orbitals must overlap. They must therefore all be aligned in the same plane.
Remember the “allyl cation” that is “stabilized by resonance”? In order for the carbocation to gain this “resonance stabilization”, the empty p orbital on the carbocation must be lined up with the adjacent pi bond.If the p orbital is at an angle of 90 degrees from the p orbitals in the pi bond, there is no conjugation and thus no resonance stabilization.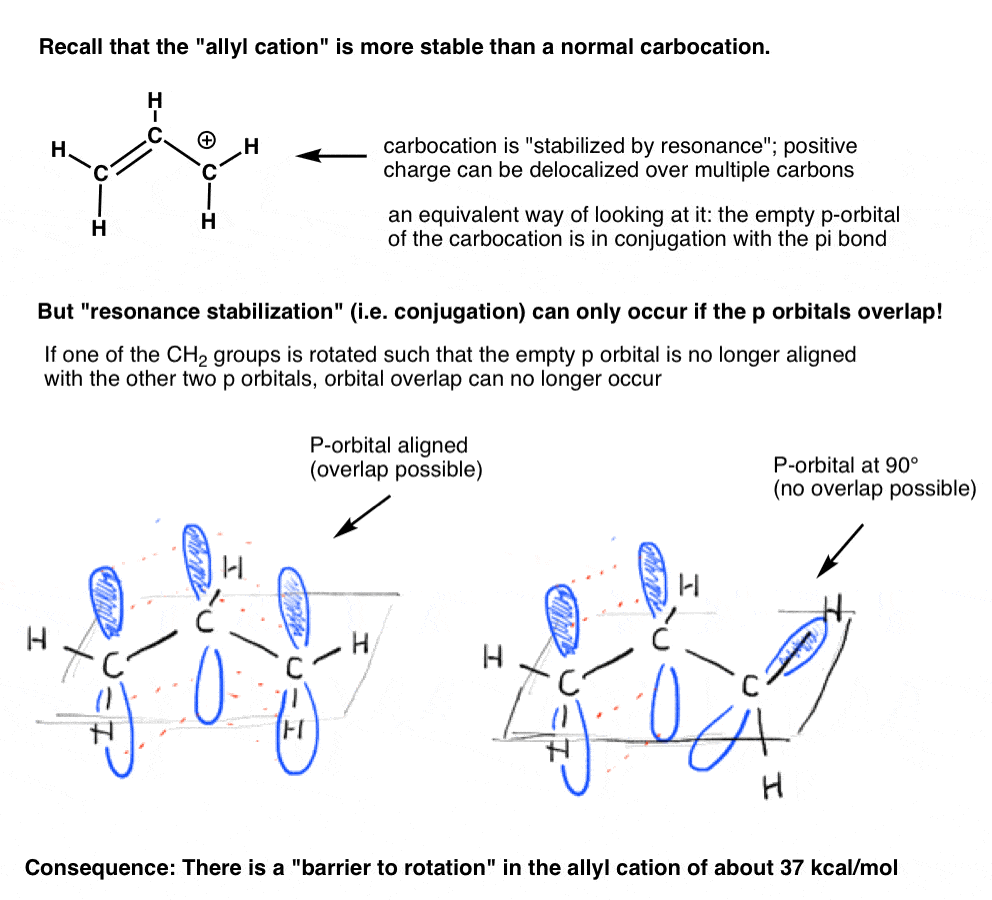
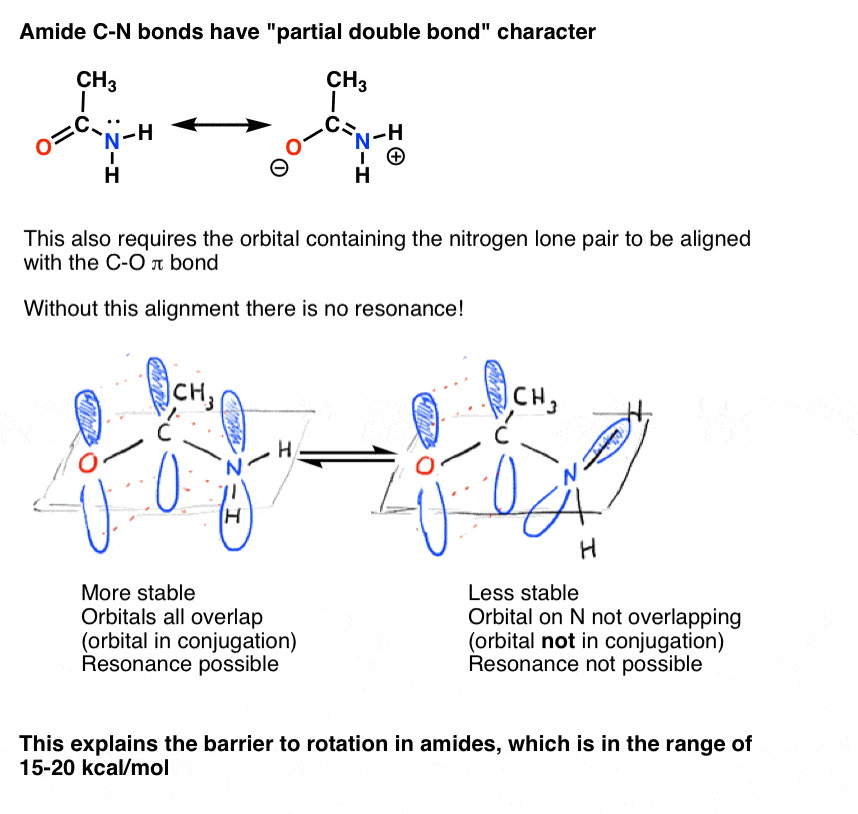
10. Bridgehead Amides Are Not Conjugated, And Are Much More Easily Broken Than “Ordinary” Amides
Bridgehead amides give an illustration of what happens to amides when overlap is impossible.Just as we saw in bridgehead alkenes, in bridgehead amides, orbital overlap between the nitrogen lone pair and carbonyl carbon is impossible due to twisting. The result is that the C-N bond does NOT have partial double bond character and it is much easier to break than a “normal” amide.The bridgehead amide below is “quinuclidone”, a twisted amide that eluded synthesis for decades. It was only in 2006 that it was finally made (as its conjugate acid) through a clever route by the lab of Brian Stoltz at Caltech.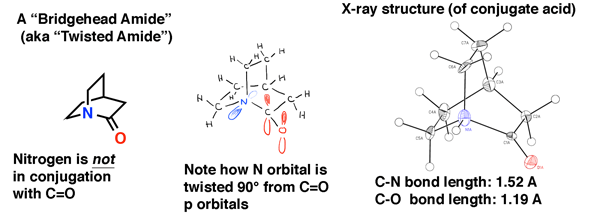
11. More Consequences Of Conjugation: Color And Cycloadditions
So far, this post has pretty much been a review of 1st semester concepts. There’s nothing in the discussion above that couldn’t be reasonably explained by what we’ve already learned about conjugation and resonance.However, this simplistic approach can only take us so far.Two quick examples, because this post has gone on long enough.First: Conjugation And ColorIf you saw this post on how bleach works, you learned that as we lengthen the conjugation length, we change the wavelength at which molecules absorb light. Some very brightly coloured molecules such as carotene, chlorophyll and lycopene all have very long conjugated double bonds.For instance, lycopene is responsible for the red colour of tomatoes. If we remove the double bonds, we remove the colour. Why?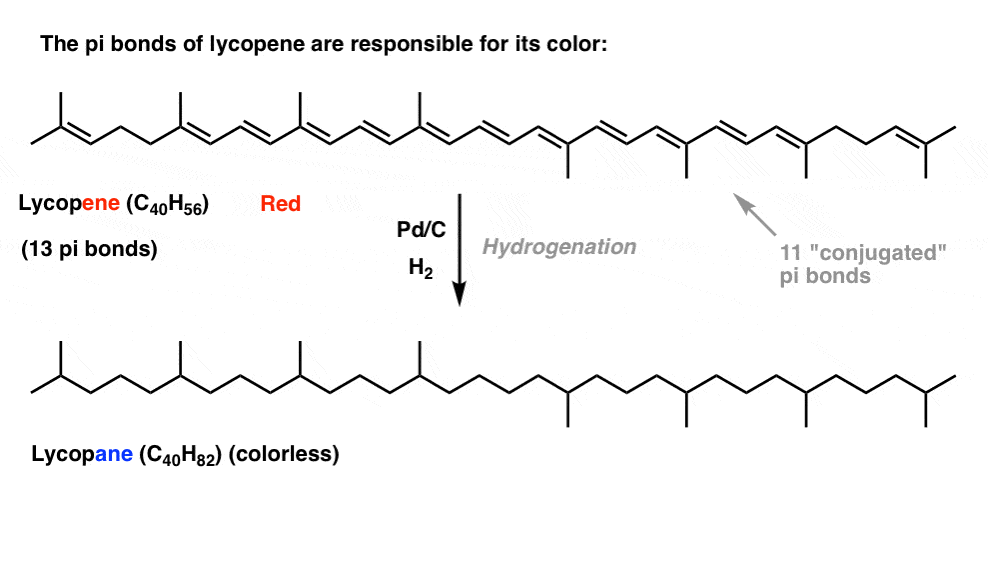
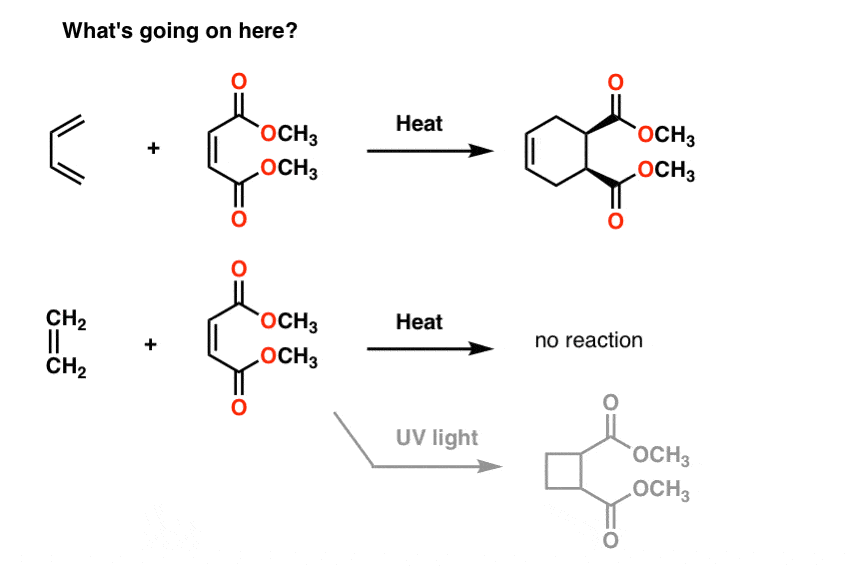
12. Next Up: Molecular Orbital Theory
What will help us answer these questions, as well as many others going forward is a concept called molecular orbital theory. In the following posts in this series, we’re going to dig more deeply into how p orbitals overlap to form molecular orbitals, and we’ll examine the energy levels of these orbitals. We’ll also see how this influences the reactivity of molecules and allows us to make predictions about their chemical behaviour.As we’ll see, molecular orbital theory provides us with a very powerful set of concepts that will help us understand chemical reactivity at a much deeper level.Thanks to Tom Struble for all his help with this post.
Notes
A-1,2 strain is the reason why tetra-tert-butylethylene has not yet been synthesized. The pi bonds are not conjugated. Look at the orbitals comprising the two pi bonds. They are at right angles to each other and cannot overlap.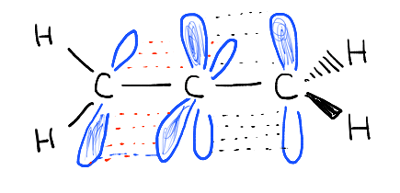
Last, Wallx.net sent you details about the topic “Conjugation And Resonance In Organic Chemistry❤️️”.Hope with useful information that the article “Conjugation And Resonance In Organic Chemistry” It will help readers to be more interested in “Conjugation And Resonance In Organic Chemistry [ ❤️️❤️️ ]”.
Posts “Conjugation And Resonance In Organic Chemistry” posted by on 2021-08-12 20:00:12. Thank you for reading the article at wallx.net