what is the hybridization of bromine in brf3
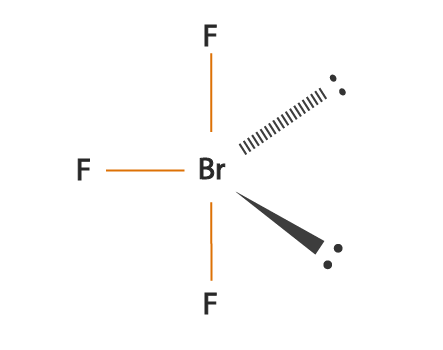
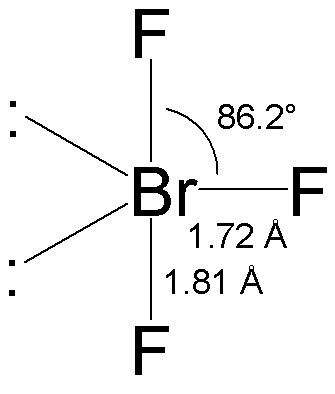
Bromine Trifluoride is often used as a strong fluoride because it is a strong binding compound. Both Bromine and Fluorine are halogens. This compound usually exists in liquid form and has a rather pungent odor. The chemical formula of this compound is BrF3. This compound was first discovered by Paul Lebeau in 1906 by performing the reaction of Bromine and fluorine at 20 degrees Celsius. It forms a T-shaped molecular structure and contains Bromine. element is the central atom. To know more about its physical properties, chemical properties and uses, it is important to first understand the geometry of the molecule and its hybridization, polarity, etc. Read: hybridization what is the chemistry of bromine in brf3 Electrons in the molecule 28 BrF3 hybridization sp3d Bond angle 86.2 degrees Molecular geometry of BrF3 Bipyramidal
Electron Geometry BrF3
Contents
BrF3 is a perfect example of an AX5 molecule having two lone electron pairs and three bonding electron pairs. Each fluorine atom has nine electrons, and there are seven valence electrons in the outermost shell of the Bromine molecule, of which three make bonds with three fluorine atoms. According to VSEPR theory, the molecular shape of the molecule should be a triangular pyramid. However, to minimize the repulsion between lone pairs, there is a bent conformation of it, giving the molecule a T shape.
Hybridization of BrF3
To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. It is represented as 1s2 2s22p6 3s23p63d104s24p5. However, to form bonds with fluorine atoms, some electrons in Bromine are transferred to the 4d orbital. This is possible because fluorine has a higher oxidizing capacity, and thus it forces Brom to promote electrons to said level. Now, Bromine can use the d orbitals for hybridization. BrF3 consists of seven electrons in its outermost shell. After bond formation, it will continue to have two lone pairs and 3 Br-F covalent bonds (bonding pairs). When the hybridization value or electron pair is 5, it gives rise to sp3d hybrid orbitals. Hence its hybridization is sp3d So the hybridization of BrF3 molecule is sp3d.
BrF3 . bond angle
Read more: what is 7 minutes in heaven The BrF3 molecular geometry is said to be T-shaped or triangular (as discussed) with a bond angle of 86.2°, slightly less than the 90° common often. The angle is formed by the repulsion of the electron pairs, which is greater than the repulsion of the Br-F bonds. [The compressed bond angles with respect to a perfect trigonal bipyramid are due to lone pairs spreading out more in space than bonded pairs.]
Is BrF3 polarized?
The Br-F bond is considered to be polar due to the relatively large difference in the electronegativity values of the fluorine and bromine atoms in the compound. Unshared pairs or lone pairs lie in the plane of the triangle, causing an uneven distribution of negative charge around the central bromine atom and thus, making the compound polar high. Hence one can say that Bromine trifluoride is polar.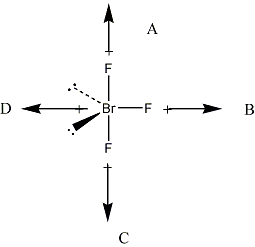
Last, Wallx.net sent you details about the topic “what is the hybridization of bromine in brf3❤️️”.Hope with useful information that the article “what is the hybridization of bromine in brf3” It will help readers to be more interested in “what is the hybridization of bromine in brf3 [ ❤️️❤️️ ]”.
Posts “what is the hybridization of bromine in brf3” posted by on 2021-09-09 14:29:13. Thank you for reading the article at wallx.net