what is the molecular geometry of pbr3
PBr3 is the chemical formula of Phosphorus Tribromide. The molecule is made up of one Phosphorus atom and three Bromine atoms. It is a colorless liquid with a pungent odor. Like PF3 and PCl3, PBr3 also exhibits properties of both Lewis acids and Lewis bases. Br3 is used in many reactions with alcohols to replace the OH functional group with a bromine atom. This reaction produces an alkyl bromide and is also known as a substitution reaction. Phosphorus Tribromide is also used to convert carboxylic acids to acyl bromides. The molecule begins to smoke when exposed to humid air due to hydrolysis Read: what is the molecular geometry of pbr3 Molecular name Phosphorus Tribromide (PBr3) Valence electron number in the molecule 26 Hybridization of PBr3 sp3 hybridization Bond angle 109.5° approx Molecular Geometry of PBr3 Triangular pyramid In this blog post we will learn about the Lewis Structure of the molecule, followed by the molecular shape, its polarity and more to better understand its chemical properties.
PBr3 Valence electrons
Contents
To understand the Lewis structure of any given molecule, we first need to know the total number of valence electrons to determine the arrangement of the atoms in the structure. bromine atom. Therefore, we need to find the valence electrons of both the Phosphorus atom and the Bromine atom. Here there are three Bromine atoms, we multiply the number by 3. Total valence electrons of PBr3 = 5 + 3 (7) = 5 + 21 = 26 There are 26 valence electrons for Phosphorus Tribromide.
PBr3 Lewis . Structure
Lewis dot structures or Lewis structures are diagrams that help in understanding the bonding of atoms along with the lone pairs present in a molecule. An atom’s valence electrons form bonds, and these bonds are represented by representing lines. Each bond occupies two valence electrons, and the excess electrons are expressed as lone pairs. Electrons that participate in bond formation are known as bonding electron pairs, while electrons that do not participate in bond formation are called lone pairs or non-bonding pairs of electrons.Read more: dubstep is what the hell To draw the Lewis structure of an atom, we need to know the following details:
- The total number of valence electrons of the molecule
- The central atom of the molecule
- The arrangement of other atoms in the molecule
Here we have learned that there are 26 valence electrons for PBr 3 and that the Bromine atom is more electronegative than the Phosphorus atom. Hence the phosphorus atom will take the center position since the least electronegative atom is placed in the center. All the bromine atoms will be arranged around the central atom like in the image below: completing the octets of the atoms placed around the central atom. The octal rule says For atoms with eight valence electrons in their outermost shells to achieve a stable structure. Here we will first try and complete the octets of all Bromine atoms, since each Bromine atom requires only one valence electron to complete its octet. Draw a line between each bromine and phosphorus. atom to represent the bond. Each bond here uses up two valence electrons; so a total of 6 valence electrons were used up in the bond formation. Since both of these atoms now have complete octets. Put other valence electrons around all the atoms, and here you have the Lewis Structure of PBr3. To confirm this structure, one can also calculate the formal charges on the atom. For this PBr3 Lewis structure, the charges on the central atom are zero, which indicates that this is a suitable Lewis dot structure for PBr3. There is a single electron pair on the central atom and three sigma bonds. in the PBr3 Lewis Structure.
Incorporation of PBr3
To find the hybridization of a given molecule, we always consider the number of hybrid orbitals formed for the bonds formed as well as the lone pairs of the molecule. As mentioned above, there are three bond pairs and one unbonded or single electron pair in Phosphorus Tribromide. The central atom forms four hybrid orbit to accommodate all four of these electron pairs. And since there are four hybrid orbitals formed, its hybrid is sp3. Finding out the steric number or hybrid orbital number is one of the simplest methods to determine the hybridization pattern of a molecule. One can also use AXN Notation method to find Hybridization Hence, PBr3 has sp3 Hybridization where there is one s and three p hybrid orbitals
PBr3 . Molecular Geometry
Read more: what is oyster surgery | The Lewis structure of molecules helps us understand the arrangement of atoms in a molecule. However, it also has some limitations. We can’t really describe the structure of a molecule in 3D, and that’s where molecular geometry helps us. Molecular geometry is the three-dimensional arrangement of atoms in a molecule that helps us to better understand the structure of a molecule. Here we use the VSEPR model to know and understand the molecular geometry of the PBr3 molecule. According to the VSEPR theory, the charge carriers repel each other and the molecules are shaped to minimize these repulsions. For example, bonding electron pairs will repel each other, and the same holds true for single electron pairs. Here in PBr3, there are 3 bonding electron pairs and a single electron pair. The molecule will have a geometry to minimize repulsion. Using the AXN notation method, we can find out the molecular geometry of the molecule. A is for the central atom, X is the number of atoms bonded to the central atom, and N is the number of pairs single on the central atom. For PBr3, the central atom is Phosphorus, three bromine atoms form a bond with it, and only a single electron pair. Therefore, it has the symbol AX3N1, and referring to the table below, we can say that it has a tetrahedral geometry.
Bond angle PBr3
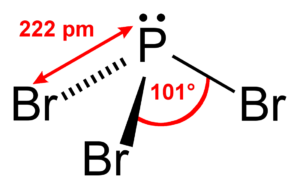
In general, the molecules have a tetrahedral electron geometry and a central atom forms a bond with three atoms with a bond angle of 109.5°. Here, however, the arrangement of the atoms is asymmetrical, and there is a single electron pair on the central atom; the bond angle won’t be exactly the same, but it will be around 109.5°.
PBr3 . Shape
The above AXN notation method also helps us to figure out the molecular shape, and for PBr3, since it has the symbol AX3N1, it has a triangular pyramidal shape.
PBr3 Pole or Non-pole
In general, molecules with asymmetrical shapes are polar in nature due to unequal charge distribution. But to confirm it, we will look at the polarity of this molecule, the electronegativity value of Phosphorus is 2.19 and that of Bromine is 2.96. The difference in electromotive force is quite high. As a result, there will be a dipole moment between the phosphorus and bromine atoms. The direction of this dipole moment will be towards the bromine atom since it is more electronegative and so the vector will be set accordingly. each other, and as a result there is a net dipole moment in the molecule. And since there is a dipole moment in this molecule, it is a polar molecule that has a partially positively charged region around the Phosphorus atom and partially negatively charged around the Bromine atom. Therefore, PBr3 or Phosphorus Tribromide is a polar molecule. blog post on PBr3, we can conclude as follows: Read more: What the stars look like in close-up
- Phosphorus Tribromide is composed of one Phosphorus atom and three Bromine atoms.
- There are a total of 26 valence electrons for PBr3.
- In the Lewis structure of PBr3, there are three bonding electron pairs and a single electron pair on the central atom.
- It has sp3 hybridization, and the bond angle is about 109.5°.
- The molecule has a triangular pyramidal shape and is a polar molecule.
Last, Wallx.net sent you details about the topic “what is the molecular geometry of pbr3❤️️”.Hope with useful information that the article “what is the molecular geometry of pbr3” It will help readers to be more interested in “what is the molecular geometry of pbr3 [ ❤️️❤️️ ]”.
Posts “what is the molecular geometry of pbr3” posted by on 2021-09-04 15:24:12. Thank you for reading the article at wallx.net