what is the molar mass of aluminum sulfate
Aluminum Sulfate: Formula
Contents
Aluminum sulfate is an ionic compound, which is a combination of negative and positive ions. And again, an ion is an atom or molecule that has a charge because it has too many or too few electrons. Also, ions are either monatomic (meaning they consist of one atom) polyatomic (meaning they are composed of more than one atom). Many of the most common ionic compounds have a positive ion consisting of a metal atom and a negative ion that is polyatomic. It’s not a rule, but it’s a useful pattern. The positive ion in aluminum sulfate is aluminum, Al + 3. The sulfate is a negative ion and a polyatomic ion (as in SO4) -2.Read: What is the molar mass of aluminum sulfate All ionic compounds must be electrically neutral , so we have to put the aluminum and sulfate ions together in the correct ratio of the electric charge to cancel. There’s a trick to determining that ratio: it’s called the “cross stitching” method. Let’s see how it works. Note that we have reversed the sign on the sulfate ion. Then all we do is put the charges as subsymbols of the opposite ion. Read more: What it says about me The formula for aluminum sulfate is Al2(SO4) 3. We must use this formula to determine the molar mass of aluminum sulfate. Let’s do that now.
Aluminum sulfate: Molar mass
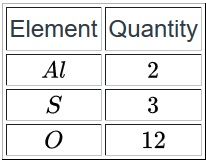
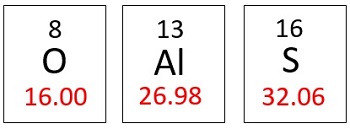
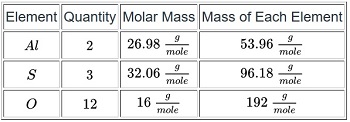
Molar mass is the mass of one mole of a substance. In this case, our substance is aluminum sulfate, so we will determine its one-molar mass. This is where the periodic table comes in. Each element on the periodic table has a molar mass listed. To get the molar mass of a compound, we add up the mass of each element in the compound. Since we have the formula for aluminum sulfate, we need to determine how many of each element make up the compound. 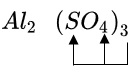
Now let’s see how aluminum sulfate is produced!
Aluminum sulfate: Production
Production of aluminum sulfate requires two steps. The first step is the chemical reaction that produces the compound, and the second step is to make the solid into particles of different sizes. Let’s start with the chemical reaction that produces it.
Chemical reaction
The chemical reaction that forms aluminum sulfate is a neutralization reaction between an acid and a base. Aluminum hydroxide (a base) is mixed with sulfuric acid (an acid) and water, creating an aluminum sulfate hydrate. It is important to remember that a hydrate is a compound that has water molecules attached to it. Read more: What are the factors of 13.
Last, Wallx.net sent you details about the topic “what is the molar mass of aluminum sulfate❤️️”.Hope with useful information that the article “what is the molar mass of aluminum sulfate” It will help readers to be more interested in “what is the molar mass of aluminum sulfate [ ❤️️❤️️ ]”.
Posts “what is the molar mass of aluminum sulfate” posted by on 2021-08-18 01:03:40. Thank you for reading the article at wallx.net

